In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model
- PMID: 16995949
- PMCID: PMC1592110
- DOI: 10.1186/1471-2407-6-226
In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model
Abstract
Background: ATP is an important signalling molecule in the peripheral and central nervous system. Both glioma growth and tumor resection induces cell death, thus liberating nucleotides to the extracellular medium. Nucleotides are hydrolyzed very slowly by gliomas when compared with astrocytes and induce neuronal cell death and glioma proliferation. The objective of the present study was to test the involvement of extracellular ATP in glioblastoma growth in a rat glioma model.
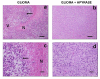
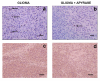
Methods: To deplete the extracellular ATP, the enzyme apyrase was tested on the treatment of gliomas implanted in the rats CNS. One million glioma C6 cells in 3 microliters of DMEM/FCS were injected in the right striata of male Wistar rats, 250-270 g. After 20 days, the rats were decapitated and the brain sectioning and stained with hematoxylin and eosine. We performed immunohistochemical experiments with Ki67, CD31 and VEGF. Total RNA was isolated from cultured glioma C6 cells and the cDNA was analyzed by Real Time-PCR with primers for the NTPDase family.
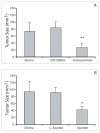
Results: C6 glioma cells effectively have a low expression of all NTPDases investigated, in comparison with normal astrocytes. The implanted glioma co-injected with apyrase had a significant reduction in the tumor size (p < 0.05) when compared with the rats injected only with gliomas or with gliomas plus inactivated apyrase. According to the pathological analysis, the malignant gliomas induced by C6 injection and co-injected with apyrase presented a significant reduction in the mitotic index and other histological characteristics that indicate a less invasive/proliferative tumor. Reduction of proliferation induced by apyrase co-injection was confirmed by counting the percentage of Ki67 positive glioma cell nuclei. According to counts with CD31, vessel density and neoformation was higher in the C6 group 20 days after implantation. Confirming this observation, rats treated with apyrase presented less VEGF staining in comparison to the control group.
Conclusion: These results indicate that the participation of extracellular ATP and the ecto-nucleotidases may be associated with the development of this type of brain tumor in an in vivo glioma model.
Figures

References
-
- Holland EC. Gliomagenesis: genetic alterations and mouse models. Nature. 2001;2:120–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

