Interaction of PAR-6 with CDC-42 is required for maintenance but not establishment of PAR asymmetry in C. elegans
- PMID: 16996049
- PMCID: PMC3148815
- DOI: 10.1016/j.ydbio.2006.08.002
Interaction of PAR-6 with CDC-42 is required for maintenance but not establishment of PAR asymmetry in C. elegans
Abstract
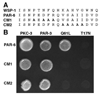
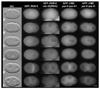
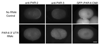
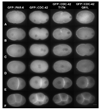
Caenorhabditis elegans embryonic polarity requires the asymmetrically distributed proteins PAR-3, PAR-6 and PKC-3. The rho family GTPase CDC-42 regulates the activities of these proteins in mammals, flies and worms. To clarify its mode of action in C. elegans we disrupted the interaction between PAR-6 and CDC-42 in vivo, and also determined the distribution of GFP-tagged CDC-42 in the early embryo. Mutant PAR-6 proteins unable to interact with CDC-42 accumulated asymmetrically, at a reduced level, but this asymmetry was not maintained during the first division. We also determined that constitutively active GFP::CDC-42 becomes enriched in the anterior during the first cell cycle in a domain that overlaps with PAR-6. The asymmetry is dependent on PAR-2, PAR-5 and PAR-6. Furthermore, we found that overexpression of constitutively active GFP::CDC-42 increased the size of the anterior domain. We conclude that the CDC-42 interaction with PAR-6 is not required for the initial establishment of asymmetry but is required for maximal cortical accumulation of PAR-6 and to maintain its asymmetry.
Figures

References
-
- Beers M, Kemphues K. Depletion of the co-chaperone CDC-37 reveals two modes of PAR-6 cortical association in C. elegans embryos. Development. 133 in press. - PubMed
-
- Boyd L, Guo S, Levitan D, Stinchcomb DT, Kemphues KJ. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development. 1996;122:3075–3084. - PubMed
-
- Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 1995;270:29071–29074. - PubMed
-
- Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. Wasp recruitment to the T cell: APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

