Intermolecular interactions of homologs of germ plasm components in mammalian germ cells
- PMID: 16996493
- PMCID: PMC2563953
- DOI: 10.1016/j.ydbio.2006.08.047
Intermolecular interactions of homologs of germ plasm components in mammalian germ cells
Abstract
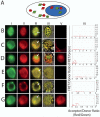
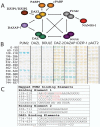
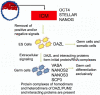
In some species such as flies, worms, frogs and fish, the key to forming and maintaining early germ cell populations is the assembly of germ plasm, microscopically distinct egg cytoplasm that is rich in RNAs, RNA-binding proteins and ribosomes. Cells which inherit germ plasm are destined for the germ cell lineage. In contrast, in mammals, germ cells are formed and maintained later in development as a result of inductive signaling from one embryonic cell type to another. Research advances, using complementary approaches, including identification of key signaling factors that act during the initial stages of germ cell development, differentiation of germ cells in vitro from mouse and human embryonic stem cells and the demonstration that homologs of germ plasm components are conserved in mammals, have shed light on key elements in the early development of mammalian germ cells. Here, we use FRET (Fluorescence Resonance Energy Transfer) to demonstrate that living mammalian germ cells possess specific RNA/protein complexes that contain germ plasm homologs, beginning in the earliest stages of development examined. Moreover, we demonstrate that, although both human and mouse germ cells and embryonic stem cells express the same proteins, germ cell-specific protein/protein interactions distinguish germ cells from precursor embryonic stem cells in vitro; interactions also determine sub-cellular localization of complex components. Finally, we suggest that assembly of similar protein complexes may be central to differentiation of diverse cell lineages and provide useful diagnostic tools for isolation of specific cell types from the assorted types differentiated from embryonic stem cells.
Figures
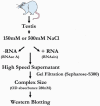
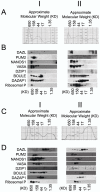
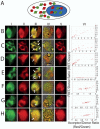
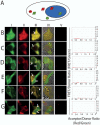

References
-
- Abeyta M, Clark AT, Rodriguez R, Bodnar M, Reijo Pera RA, Firpo M. Unique gene expression signatures of independently-derived human embryonic stem cell lines. Hum Mol Genet. 2004;13:601–608. - PubMed
-
- Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nature Cell Biol. 1999;1:431–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

