Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic
- PMID: 16997913
- PMCID: PMC1595449
- DOI: 10.1073/pnas.0606176103
Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic
Erratum in
- Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16614
-
Correction for Kuroda et al., Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic.Proc Natl Acad Sci U S A. 2025 Aug 26;122(34):e2519923122. doi: 10.1073/pnas.2519923122. Epub 2025 Aug 20. Proc Natl Acad Sci U S A. 2025. PMID: 40833421 Free PMC article. No abstract available.
Abstract
Cell killing is a critical pharmacological activity of imatinib to eradicate Bcr/Abl+ leukemias. We found that imatinib kills Bcr/Abl+ leukemic cells by triggering the Bcl-2-regulated apoptotic pathway. Imatinib activated several proapoptotic BH3-only proteins: bim and bmf transcription was increased, and both Bim and Bad were activated posttranslationally. Studies using RNAi and cells from gene-targeted mice revealed that Bim plays a major role in imatinib-induced apoptosis of Bcr/Abl+ leukemic cells and that the combined loss of Bim and Bad abrogates this killing. Loss of Bmf or Puma had no effect. Resistance to imatinib caused by Bcl-2 overexpression or loss of Bim (plus Bad) could be overcome by cotreatment with the BH3 mimetic ABT-737. These results demonstrate that Bim and Bad account for most, perhaps all, imatinib-induced killing of Bcr/Abl+ leukemic cells and suggest previously undescribed drug combination strategies for cancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
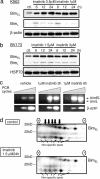
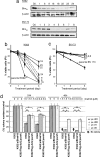
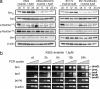
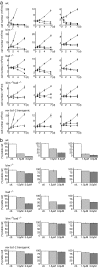

Comment in
-
Imatinib spells BAD news for Bcr/abl-positive leukemias.Proc Natl Acad Sci U S A. 2006 Oct 3;103(40):14651-2. doi: 10.1073/pnas.0607135103. Epub 2006 Sep 26. Proc Natl Acad Sci U S A. 2006. PMID: 17003111 Free PMC article. No abstract available.
References
-
- Deininger M, Buchdunger E, Druker BJ. Blood. 2005;105:2640–2653. - PubMed
-
- Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, Forman SJ. Blood. 2003;101:4701–4707. - PubMed
-
- Strasser A, O'Connor L, Dixit VM. Annu Rev Biochem. 2000;69:217–245. - PubMed
-
- Green DR, Kroemer G. Science. 2004;305:626–629. - PubMed
-
- Puthalakath H, Strasser A. Cell Death Differ. 2002;9:505–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

