Exogenous free ubiquitin enhances lily pollen tube adhesion to an in vitro stylar matrix and may facilitate endocytosis of SCA
- PMID: 16998086
- PMCID: PMC1676050
- DOI: 10.1104/pp.106.086801
Exogenous free ubiquitin enhances lily pollen tube adhesion to an in vitro stylar matrix and may facilitate endocytosis of SCA
Abstract
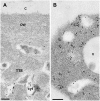
Pollen tube adhesion and guidance on extracellular matrices within the pistil are essential processes that convey the pollen tube cell and the sperm cells to the ovule. In this study, we purified an additional molecule from the pistil that enhances pollen tube adhesion when combined with the SCA (stigma/stylar cysteine-rich adhesin)/pectin matrix in our in vitro assay. The enhancer of adhesion was identified as free ubiquitin (Ub). This was confirmed by use of bovine Ub as a substitute for lily (Lilium longiflorum Thunb.) stigma Ub. To study the interaction of SCA and Ub with the lily pollen tube, we labeled both proteins with biotin. We observed uptake of biotin-labeled SCA and Ub into the pollen tube cells in vitro using confocal microscopy. For SCA, a strong signal occurred first at the tip of the pollen tube, suggestive of an endocytosis event, and then progressively throughout the tube cytoplasm. SCA was also localized inside the in vivo pollen tube using immunogold electron microscopy and found to be present in endosomes, multivesicular bodies, and vacuoles, all known to be endocytic compartments. It was also confirmed that SCA is endocytosed in the in vitro adhesion assay. Internalization of SCA was increased in pollen tubes treated with exogenous Ub compared to those without Ub, suggesting that Ub may facilitate SCA endocytosis. These results show that Ub can act as an enhancer of pollen tube adhesion in vitro and that it is taken up into the pollen tube as is SCA. The Ub machinery may play a role in pollen tube adhesion and guidance in lily.
Figures










References
-
- Bahaji A, Cornejo MJ, Ortiz-Zapater E, Contreras I, Aniento F (2001) Uptake of endocytic markers by rice cells: variations related to the growth phase. Eur J Cell Biol 80: 178–186 - PubMed
-
- Beers EP, Moreno TM, Callis J (1992) Subcellular localization of ubiquitin and ubiquitinated proteins in Arabidopsis thaliana. J Biochem (Tokyo) 267: 15432–15439 - PubMed
-
- Blackbourn HD, Jackson AP (1996) Plant clathrin heavy chain: sequence analysis and restricted localisation in growing pollen tubes. J Cell Sci 109: 777–787 - PubMed
-
- Blein JP, Coutos-Thevenot P, Marion D, Ponchet M (2002) From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci 7: 293–296 - PubMed
-
- Buhot N, Douliez JP, Jacquemard A, Marion D, Tran V, Maume BF, Milat ML, Ponchet M, Mikes V, Kader JC, et al (2001) A lipid transfer protein binds to a receptor involved in the control of plant defence responses. FEBS Lett 509: 27–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

