Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease
- PMID: 16998589
- PMCID: PMC1570376
- DOI: 10.1172/JCI27798
Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease
Erratum in
- J Clin Invest. 2008 May;118(5):1974
Abstract
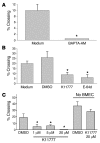
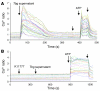
In this study we investigated why bloodstream forms of Trypanosoma brucei gambiense cross human brain microvascular endothelial cells (BMECs), a human blood-brain barrier (BBB) model system, at much greater efficiency than do T. b. brucei. After noting that T. b. gambiense displayed higher levels of cathepsin L-like cysteine proteases, we investigated whether these enzymes contribute to parasite crossing. First, we found that T. b. gambiense crossing of human BMECs was abrogated by N-methylpiperazine-urea-Phe-homopheylalanine-vinylsulfone-benzene (K11777), an irreversible inhibitor of cathepsin L-like cysteine proteases. Affinity labeling and immunochemical studies characterized brucipain as the K11777-sensitive cysteine protease expressed at higher levels by T. b. gambiense. K11777-treated T. b. gambiense failed to elicit calcium fluxes in BMECs, suggesting that generation of activation signals for the BBB is critically dependant on brucipain activity. Strikingly, crossing of T. b. brucei across the BBB was enhanced upon incubation with brucipain-rich supernatants derived from T. b. gambiense. The effects of the conditioned medium, which correlated with ability to evoke calcium fluxes, were canceled by K11777, but not by the cathepsin B inhibitor CA074. Collectively, these in vitro studies implicate brucipain as a critical driver of T. b. gambiense transendothelial migration of the human BBB.
Figures




References
-
- Naessens J. Bovine trypanotolerance: a natural ability to prevent severe anaemia and haemophagocytic syndrome? Int. J. Parasitol. 2006;36:521–528. - PubMed
-
- Grab D.J, et al. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J. Parasitol. 2004;90:970–979. - PubMed
-
- Dumas M., Bouteille B. Current status of trypanosomiasis. Med. Trop. (Mars). 1997;57:65–69. - PubMed
-
- Lonsdale-Eccles J.D., Grab D.J. Trypanosome hydrolases and the blood-brain barrier. Trends Parasitol. 2002;18:17–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

