Substituent effects on Gd(III)-based MRI contrast agents: optimizing the stability and selectivity of the complex and the number of coordinated water molecules
- PMID: 16999435
- PMCID: PMC3190981
- DOI: 10.1021/ic061262q
Substituent effects on Gd(III)-based MRI contrast agents: optimizing the stability and selectivity of the complex and the number of coordinated water molecules
Abstract
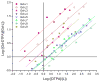
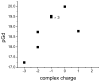
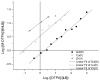
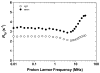
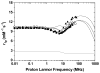
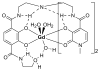
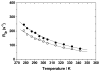
Hydroxypyridinone (HOPO)-based Gd(III) complexes have previously been shown to exhibit high relaxivity, especially at the high magnetic fields that are clinically relevant for present and future clinical use. This is due to more than one coordinated water molecule exchanging rapidly with bulk solvent. These complexes, however, present poor water solubility. Heteropodal complexes which include a terephthalamide (TAM) moiety maintain the high relaxivity characteristics of the HOPO family and have been functionalized with solubilizing substituents of various charges. The charge of the substituent significantly affects the stability of the Gd(III) complex, with the most stable complex presenting a neutral charge. The solubilizing substituent also moderately affects the affinity of the complex for physiological anions, with the highest affinity observed for the positively charged complex. In any case, only two anions, phosphate and oxalate, measureably bind the Gd(III) complex with weak affinities that are comparable to other q = 1 complexes and much weaker than DO3A, q = 2 based complexes. Furthermore, unlike poly(amino-carboxylate)-based complexes, HOPO-based Gd(III) complexes do not show any noticeable interaction with carbonates. The nature of the substituent can also favorably stabilize the coordination of a third water molecule on the Gd(III) center and lead to a nine-coordinate ground state. Such complexes that attain q = 3 incorporate a substituent beta to the terminal amide of the TAM podand that is a hydrogen-bond acceptor, suggesting that the third water molecule is coordinated to the metal center through a hydrogen-bond network. These substituents include alcohols, primary amines, and acids. Moreover, the coordination of a third water molecule has been achieved without destabilizing the complex.
Figures

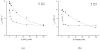






References
-
-
Paper no. 19 in the series “High Relaxivity Gadolinium MRI Agents”; for the previous paper in the series see Puerta DT, Botta M, Jocher CJ, Werner EJ, Avedano S, Raymond KN, Cohen SM. J Am Chem Soc. 2006;128:2222–2223.
-
-
- Merbach AE, Tóth E. Wiley. The Chemistry of Contrast Agents. Chichester: 2001.
-
- Laus S, Ruloff R, Toth E, Merbach AE. Chem-Eur J. 2003;9:3555–3566. - PubMed
-
- Ruloff R, Toth E, Scopelliti R, Tripier R, Handel H, Merbach AE. Chem Comm. 2002:2630–2631. - PubMed
-
- Wang YM, Lee C, Liu Hl, Sheu GCRS. Dalton Trans. 1998;24:4113–4118.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

