Evolutionarily conserved role of nucleostemin: controlling proliferation of stem/progenitor cells during early vertebrate development
- PMID: 17000755
- PMCID: PMC1698517
- DOI: 10.1128/MCB.01183-06
Evolutionarily conserved role of nucleostemin: controlling proliferation of stem/progenitor cells during early vertebrate development
Abstract
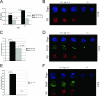
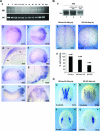
Nucleostemin (NS) is a putative GTPase expressed preferentially in the nucleoli of neuronal and embryonic stem cells and several cancer cell lines. Transfection and knockdown studies indicated that NS controls the proliferation of these cells by interacting with the p53 tumor suppressor protein and regulating its activity. To assess the physiological role of NS in vivo, we generated a mutant mouse line with a specific gene trap event that inactivates the NS allele. The corresponding NS(-/-) embryos died around embryonic day 4. Analyses of NS mutant blastocysts indicated that NS is not required to maintain pluripotency, nucleolar integrity, or survival of the embryonic stem cells. However, the homozygous mutant blastocysts failed to enter S phase even in the absence of functional p53. Haploid insufficiency of NS in mouse embryonic fibroblasts leads to decreased cell proliferation. NS also functions in early amphibian development to control cell proliferation of neural progenitor cells. Our results show that NS has a unique ability, derived from an ancestral function, to control the proliferation rate of stem/progenitor cells in vivo independently of p53.
Figures




References
-
- Artus, J., C. Babinet, and M. Cohen-Tannoudji. 2006. The cell cycle of early mammalian embryos: lessons from genetic mouse models. Cell Cycle 5:499-502. - PubMed
-
- Bassler J., P. Grandi, O. Gadal, T. Lessmann, E. Petfalski, D. Tollervey, J. Lechner, and E. Hurt. 2001. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell 8:517-529. - PubMed
-
- Bellmeyer, A., J. Krase, J. Lindgren, and C. LaBonne. 2003. The protooncogene c-myc is an essential regulator of neural crest formation in xenopus. Dev. Cell 4:827-839. - PubMed
-
- Bernardi, R., and P. P. Pandolfi. 2003. The nucleolus: at the stem of immortality. Nat. Med. 9:24-25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
