The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression
- PMID: 17000761
- PMCID: PMC1636837
- DOI: 10.1128/MCB.02299-05
The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression
Abstract
Commitment to the melanocyte lineage is characterized by the onset of expression of the microphthalmia-associated transcription factor (Mitf). This transcription factor plays a fundamental role in melanocyte development and maintenance and seems to be crucial for the survival of malignant melanocytes. Furthermore, Mitf has been shown to be involved in cell cycle regulation and to play important functions in self-renewal and maintenance of melanocyte stem cells. Although little is known about how Mitf regulates these various processes, one possibility is that Mitf interacts with other regulators. Here we show that Mitf can interact directly with beta-catenin, the key mediator of the canonical Wnt signaling pathway. The Wnt signaling pathway plays a critical role in melanocyte development and is intimately involved in triggering melanocyte stem cell proliferation. Significantly, constitutive activation of this pathway is a feature of a number of cancers including malignant melanoma. Here we show that Mitf can redirect beta-catenin transcriptional activity away from canonical Wnt signaling-regulated genes toward Mitf-specific target promoters to activate transcription. Thus, by a feedback mechanism, Mitf can diversify the output of canonical Wnt signaling to enhance the repertoire of genes regulated by beta-catenin. Our results reveal a novel mechanism by which Wnt signaling and beta-catenin activate gene expression, with significant implications for our understanding of both melanocyte development and melanoma.
Figures
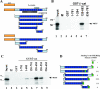


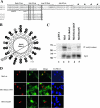
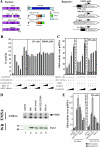


References
-
- Aberle, H., S. Butz, J. Stappert, H. Weissig, R. Kemler, and H. Hoschuetzky. 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107:3655-3663. - PubMed
-
- Behrens, J., B. A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3β. Science 280:596-599. - PubMed
-
- Bek, S., and R. Kemler. 2002. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J. Cell Sci. 115:4743-4753. - PubMed
-
- Bienz, M. 2005. β-Catenin: a pivot between cell adhesion and Wnt signalling. Curr. Biol. 15:R64-R67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
