The Hsp90 cochaperone p23 is essential for perinatal survival
- PMID: 17000766
- PMCID: PMC1636834
- DOI: 10.1128/MCB.00734-06
The Hsp90 cochaperone p23 is essential for perinatal survival
Abstract
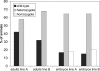
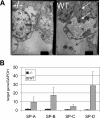
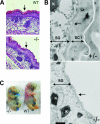
The functions of molecular chaperones have been extensively investigated biochemically in vitro and genetically in bacteria and yeast. We have embarked on a functional genomic analysis of the Hsp90 chaperone machine in the mouse by disrupting the p23 gene using a gene trap approach. p23 is an Hsp90 cochaperone that is thought to stabilize Hsp90-substrate complexes and, independently, to act as the cytosolic prostaglandin E2 synthase. Gene deletions in budding and fission yeasts and knock-down experiments with the worm have not revealed any clear in vivo requirements for p23. We find that p23 is not essential for overall prenatal development and morphogenesis of the mouse, which parallels the observation that it is dispensable for proliferation in yeast. In contrast, p23 is absolutely necessary for perinatal survival. Apart from an incompletely formed skin barrier, the lungs of p23 null embryos display underdeveloped airspaces and substantially reduced expression of surfactant genes. Correlating with the known function of glucocorticoids in promoting lung maturation and the role of p23 in the assembly of a hormone-responsive glucocorticoid receptor-Hsp90 complex, p23 null fibroblast cells have a defective glucocorticoid response. Thus, p23 contributes a nonredundant, temporally restricted, and tissue-specific function during mouse development.
Figures
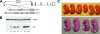

References
-
- Acarregui, M. J., J. M. Snyder, M. D. Mitchell, and C. R. Mendelson. 1990. Prostaglandins regulate surfactant protein A (SP-A) gene expression in human fetal lung in vitro. Endocrinology 127:1105-1113. - PubMed
-
- Bose, S., T. Weikl, H. Bügl, and J. Buchner. 1996. Chaperone function of Hsp90-associated proteins. Science 274:1715-1717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
