Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts
- PMID: 17000866
- PMCID: PMC2118097
- DOI: 10.1084/jem.20060845
Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts
Abstract
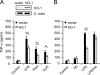
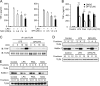
Carbon monoxide (CO), a byproduct of heme catabolism by heme oxygenase (HO), confers potent antiinflammatory effects. Here we demonstrate that CO derived from HO-1 inhibited Toll-like receptor (TLR) 2, 4, 5, and 9 signaling, but not TLR3-dependent signaling, in macrophages. Ligand-mediated receptor trafficking to lipid rafts represents an early event in signal initiation of immune cells. Trafficking of TLR4 to lipid rafts in response to LPS was reactive oxygen species (ROS) dependent because it was inhibited by diphenylene iodonium, an inhibitor of NADPH oxidase, and in gp91(phox)-deficient macrophages. CO selectively inhibited ligand-induced recruitment of TLR4 to lipid rafts, which was also associated with the inhibition of ligand-induced ROS production in macrophages. TLR3 did not translocate to lipid rafts by polyinosine-polycytidylic acid (poly(I:C)). CO had no effect on poly(I:C)-induced ROS production and TLR3 signaling. The inhibitory effect of CO on TLR-induced cytokine production was abolished in gp91(phox)-deficient macrophages, also indicating a role for NADPH oxidase. CO attenuated LPS-induced NADPH oxidase activity in vitro, potentially by binding to gp91(phox). Thus, CO negatively controlled TLR signaling pathways by inhibiting translocation of TLR to lipid rafts through suppression of NADPH oxidase-dependent ROS generation.
Figures


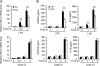




Similar articles
-
Soyasaponin Bb inhibits the recruitment of toll-like receptor 4 (TLR4) into lipid rafts and its signaling pathway by suppressing the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent generation of reactive oxygen species.Mol Nutr Food Res. 2016 Jul;60(7):1532-43. doi: 10.1002/mnfr.201600015. Epub 2016 Apr 21. Mol Nutr Food Res. 2016. PMID: 27005845
-
Lycopene suppresses proinflammatory response in lipopolysaccharide-stimulated macrophages by inhibiting ROS-induced trafficking of TLR4 to lipid raft-like domains.J Nutr Biochem. 2013 Jun;24(6):1117-22. doi: 10.1016/j.jnutbio.2012.08.011. Epub 2012 Dec 14. J Nutr Biochem. 2013. PMID: 23246157
-
Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol.J Lipid Res. 2010 Nov;51(11):3196-206. doi: 10.1194/jlr.M006486. Epub 2010 Jul 21. J Lipid Res. 2010. PMID: 20650929 Free PMC article.
-
Carbon monoxide - beyond toxicity?Toxicol Lett. 2020 Oct 15;333:251-260. doi: 10.1016/j.toxlet.2020.08.010. Epub 2020 Aug 26. Toxicol Lett. 2020. PMID: 32860873 Review.
-
Role of lipid microdomains in TLR-mediated signalling.Biochim Biophys Acta. 2015 Sep;1848(9):1860-7. doi: 10.1016/j.bbamem.2015.03.014. Epub 2015 Mar 20. Biochim Biophys Acta. 2015. PMID: 25797518 Review.
Cited by
-
IFNγ causes mitochondrial dysfunction and oxidative stress in myositis.Nat Commun. 2024 Jun 26;15(1):5403. doi: 10.1038/s41467-024-49460-1. Nat Commun. 2024. PMID: 38926363 Free PMC article.
-
Toll-like receptor 4 is not targeted to the lysosome in cystic fibrosis airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2013 Mar 1;304(5):L371-82. doi: 10.1152/ajplung.00372.2011. Epub 2013 Jan 11. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23316065 Free PMC article.
-
Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease.Kidney Int. 2013 Jun;83(6):1029-41. doi: 10.1038/ki.2012.439. Epub 2013 Jan 16. Kidney Int. 2013. PMID: 23325084 Free PMC article. Review.
-
Myeloid cell-specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis.J Clin Invest. 2013 Apr;123(4):1784-97. doi: 10.1172/JCI60415. Epub 2013 Mar 15. J Clin Invest. 2013. PMID: 23549085 Free PMC article.
-
Deciphering the Role of Heme Oxygenase-1 (HO-1) Expressing Macrophages in Renal Ischemia-Reperfusion Injury.Biomedicines. 2021 Mar 16;9(3):306. doi: 10.3390/biomedicines9030306. Biomedicines. 2021. PMID: 33809696 Free PMC article. Review.
References
-
- Ryter, S.W., D. Morse, and A.M. Choi. 2004. Carbon monoxide: to boldly go where NO has gone before. Sci. STKE. 2004:RE6. - PubMed
-
- Kawashima, A., Y. Oda, A. Yachie, S. Koizumi, and I. Nakanishi. 2002. Heme oxygenase-1 deficiency: the first autopsy case. Hum. Pathol. 33:125–130. - PubMed
-
- Otterbein, L.E., F.H. Bach, J. Alam, M. Soares, H. Tao Lu, M. Wysk, R.J. Davis, R.A. Flavell, and A.M. Choi. 2000. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 6:422–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

