A novel role for phagocytosis-like uptake in herpes simplex virus entry
- PMID: 17000878
- PMCID: PMC2064392
- DOI: 10.1083/jcb.200509155
A novel role for phagocytosis-like uptake in herpes simplex virus entry
Abstract
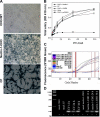
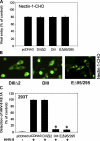
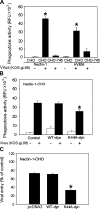
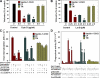
It is becoming increasingly clear that herpesviruses can exploit the endocytic pathway to infect cells, yet several important features of this process remain poorly defined. Using herpes simplex virus-1 (HSV-1) as a model, we demonstrate that endocytosis of the virions mimic many features of phagocytosis. During entry, HSV-1 virions associated with plasma membrane protrusions followed by a phagocytosis-like uptake involving rearrangement of actin cytoskeleton and trafficking of the virions in large phagosome-like vesicles. RhoA GTPase was activated during this process and the mode of entry was cell type-specific. Clathrin-coated vesicles had no detectable role in virion trafficking as Eps15 dominant-negative mutants failed to affect HSV-1 uptake. Binding and fusion of the virion envelope with the phagosomal membrane is likely facilitated by clustering of nectin-1 (or HVEM) in phagosomes, which was observed in infected cells. Collectively, our data suggests a novel mode of uptake by which the virus can infect both professional and nonprofessional phagocytes.
Figures



References
-
- Amer, A.O., and M.S. Swanson. 2002. A phagosome of one's own: a microbial guide to life in the macrophage. Curr. Opin. Microbiol. 5:56–61. - PubMed
-
- Benmerah, A., B. Bègue, A. Dautry-Varsat, and N. Cerf-Bensussan. 1996. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J. Biol. Chem. 271:12111–12116. - PubMed
-
- Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303–1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

