Hormonal activity of AIMP1/p43 for glucose homeostasis
- PMID: 17001013
- PMCID: PMC1595450
- DOI: 10.1073/pnas.0602045103
Hormonal activity of AIMP1/p43 for glucose homeostasis
Abstract
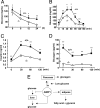
AIMP1/p43 is known as a cytokine working in the control of angiogenesis, inflammation, and wound healing. Here we report its enrichment in pancreatic alpha cells and glucagon-like hormonal activity. AIMP1 is secreted from the pancreas upon glucose starvation. Exogenous infusion of AIMP1 increased plasma levels of glucose, glucagon, and fatty acid, and AIMP1-deficient mice showed reduced plasma glucose levels compared with the wild-type mice under fasting conditions. Thus, AIMP1 plays a glucagon-like role in glucose homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

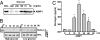


Similar articles
-
AIMP1 deficiency enhances airway hyperreactivity in mice via increased TH2 immune responses.Clin Immunol. 2012 Jun;143(3):256-65. doi: 10.1016/j.clim.2012.02.004. Epub 2012 Mar 3. Clin Immunol. 2012. PMID: 22472603
-
Aminoacyl-tRNA synthetase-interacting multi-functional protein 1/p43: an emerging therapeutic protein working at systems level.Expert Opin Drug Discov. 2008 Aug;3(8):945-57. doi: 10.1517/17460441.3.8.945. Expert Opin Drug Discov. 2008. PMID: 23484969
-
Inhibition of glucagon secretion.Adv Pharmacol. 2005;52:151-71. doi: 10.1016/S1054-3589(05)52008-8. Adv Pharmacol. 2005. PMID: 16492545 Review.
-
Glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse.Gastroenterology. 2010 Sep;139(3):857-68. doi: 10.1053/j.gastro.2010.05.006. Epub 2010 Jun 11. Gastroenterology. 2010. PMID: 20546737
-
Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes.J Endocrinol. 2008 Oct;199(1):5-19. doi: 10.1677/JOE-08-0290. Epub 2008 Jul 31. J Endocrinol. 2008. PMID: 18669612 Review.
Cited by
-
AIMP1 promotes multiple myeloma malignancy through interacting with ANP32A to mediate histone H3 acetylation.Cancer Commun (Lond). 2022 Nov;42(11):1185-1206. doi: 10.1002/cac2.12356. Epub 2022 Aug 30. Cancer Commun (Lond). 2022. PMID: 36042007 Free PMC article.
-
Structure of the ArgRS-GlnRS-AIMP1 complex and its implications for mammalian translation.Proc Natl Acad Sci U S A. 2014 Oct 21;111(42):15084-9. doi: 10.1073/pnas.1408836111. Epub 2014 Oct 6. Proc Natl Acad Sci U S A. 2014. PMID: 25288775 Free PMC article.
-
Liver Patt1 deficiency protects male mice from age-associated but not high-fat diet-induced hepatic steatosis.J Lipid Res. 2012 Mar;53(3):358-367. doi: 10.1194/jlr.M019257. Epub 2012 Jan 9. J Lipid Res. 2012. PMID: 22231784 Free PMC article.
-
Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ.J Lipid Res. 2011 May;52(5):908-22. doi: 10.1194/jlr.M013375. Epub 2011 Feb 22. J Lipid Res. 2011. PMID: 21343633 Free PMC article.
-
A novel endogenous damage signal, glycyl tRNA synthetase, activates multiple beneficial functions of mesenchymal stem cells.Cell Death Differ. 2018 Nov;25(11):2023-2036. doi: 10.1038/s41418-018-0099-2. Epub 2018 Apr 17. Cell Death Differ. 2018. PMID: 29666468 Free PMC article.
References
-
- Deutscher MP. Methods Enzymol. 1974;29:577–583. - PubMed
-
- Dang CV, Yang DC. Int J Biochem. 1982;14:539–543. - PubMed
-
- Yang DC, Garcia JV, Johnson YD, Wahab S. Curr Top Cell Regul. 1985;26:325–335. - PubMed
-
- Park SG, Jung KH, Lee JS, Jo YJ, Motegi H, Kim S, Shiba K. J Biol Chem. 1999;274:16673–16676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

