Cyclic nucleotide-gated ion channels in rod photoreceptors are protected from retinoid inhibition
- PMID: 17001087
- PMCID: PMC2151575
- DOI: 10.1085/jgp.200609619
Cyclic nucleotide-gated ion channels in rod photoreceptors are protected from retinoid inhibition
Abstract
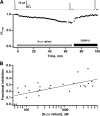
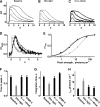
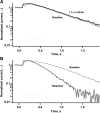
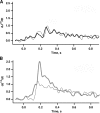
In vertebrate rods, photoisomerization of the 11-cis retinal chromophore of rhodopsin to the all-trans conformation initiates a biochemical cascade that closes cGMP-gated channels and hyperpolarizes the cell. All-trans retinal is reduced to retinol and then removed to the pigment epithelium. The pigment epithelium supplies fresh 11-cis retinal to regenerate rhodopsin. The recent discovery that tens of nanomolar retinal inhibits cloned cGMP-gated channels at low [cGMP] raised the question of whether retinoid traffic across the plasma membrane of the rod might participate in the signaling of light. Native channels in excised patches from rods were very sensitive to retinoid inhibition. Perfusion of intact rods with exogenous 9- or 11-cis retinal closed cGMP-gated channels but required higher than expected concentrations. Channels reopened after perfusing the rod with cellular retinoid binding protein II. PDE activity, flash response kinetics, and relative sensitivity were unchanged, ruling out pharmacological activation of the phototransduction cascade. Bleaching of rhodopsin to create all-trans retinal and retinol inside the rod did not produce any measurable channel inhibition. Exposure of a bleached rod to 9- or 11-cis retinal did not elicit channel inhibition during the period of rhodopsin regeneration. Microspectrophotometric measurements showed that exogenous 9- or 11-cis retinal rapidly cross the plasma membrane of bleached rods and regenerate their rhodopsin. Although dark-adapted rods could also take up large quantities of 9-cis retinal, which they converted to retinol, the time course was slow. Apparently cGMP-gated channels in intact rods are protected from the inhibitory effects of retinoids that cross the plasma membrane by a large-capacity buffer. Opsin, with its chromophore binding pocket occupied (rhodopsin) or vacant, may be an important component. Exceptionally high retinoid levels, e.g., associated with some retinal degenerations, could overcome the buffer, however, and impair sensitivity or delay the recovery after exposure to bright light.
Figures

Similar articles
-
All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: a novel role for photoreceptor retinoids in the response to bright light?Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):8372-7. doi: 10.1073/pnas.122681899. Epub 2002 May 28. Proc Natl Acad Sci U S A. 2002. PMID: 12034887 Free PMC article.
-
Defining the retinoid binding site in the rod cyclic nucleotide-gated channel.J Gen Physiol. 2005 Nov;126(5):453-60. doi: 10.1085/jgp.200509387. Epub 2005 Oct 17. J Gen Physiol. 2005. PMID: 16230468 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
All-trans-retinal is a closed-state inhibitor of rod cyclic nucleotide-gated ion channels.J Gen Physiol. 2004 May;123(5):521-31. doi: 10.1085/jgp.200409011. Epub 2004 Apr 12. J Gen Physiol. 2004. PMID: 15078915 Free PMC article.
-
Vitamin A and Vision.Subcell Biochem. 2016;81:231-259. doi: 10.1007/978-94-024-0945-1_9. Subcell Biochem. 2016. PMID: 27830507 Review.
Cited by
-
Signalling beyond photon absorption: extracellular retinoids and growth factors modulate rod photoreceptor sensitivity.J Physiol. 2016 Apr 1;594(7):1841-54. doi: 10.1113/JP271650. Epub 2016 Jan 23. J Physiol. 2016. PMID: 26691896 Free PMC article.
-
Vitamin A activates rhodopsin and sensitizes it to ultraviolet light.Vis Neurosci. 2011 Nov;28(6):485-97. doi: 10.1017/S0952523811000423. Vis Neurosci. 2011. PMID: 22192505 Free PMC article.
-
Beta-ionone activates and bleaches visual pigment in salamander photoreceptors.Vis Neurosci. 2009 May-Jun;26(3):267-74. doi: 10.1017/S0952523809090105. Epub 2009 Jun 5. Vis Neurosci. 2009. PMID: 19500430 Free PMC article.
-
Turning cones off: the role of the 9-methyl group of retinal in red cones.J Gen Physiol. 2006 Dec;128(6):671-85. doi: 10.1085/jgp.200609630. Epub 2006 Nov 13. J Gen Physiol. 2006. PMID: 17101818 Free PMC article.
References
-
- Allikmets, R., N. Singh, H. Sun, N.F. Shroyer, A. Hutchinson, A. Chidambaram, B. Gerrard, L. Baird, D. Stauffer, A. Peiffer, et al. 1997. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat. Genet. 15:236–246. - PubMed
-
- Brown, P.K., and G. Wald. 1956. The neo-b isomer of vitamin A and retinene. J. Biol. Chem. 222:865–877. - PubMed
-
- Cohen, G.B., D.D. Oprian, and P.R. Robinson. 1992. Mechanism of activation and inactivation of opsin: role of Glu113 and Lys296. Biochemistry. 31:12592–12601. - PubMed
-
- Cornwall, M.C., G.J. Jones, V.J. Kefalov, G.L. Fain, and H.R. Matthews. 2000. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors. Methods Enzymol. 316:224–252. - PubMed

