RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75
- PMID: 17001303
- PMCID: PMC2014681
- DOI: 10.1038/sj.bjp.0706909
RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75
Abstract
Background and purpose: RANTES is an inflammatory chemokine with a critical role in T-lymphocyte activation and proliferation. Its effects are mediated through G protein-coupled heptahelical receptors (GPCRs). We show for the first time that RANTES activates the orphan G protein-coupled receptor 75 (GPR75).
Experimental approach: To identify a ligand for GPR75 we have used three different and independent methods, namely luciferase assay, bioluminescence assay and IP3 accumulation assay.
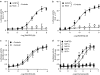
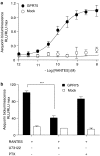
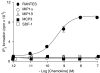
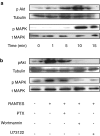
Key results: Treatment of cells expressing GPR75 with subnanomolar concentrations of RANTES led to stimulation of the luciferase activity in a reporter-gene assay, an increase in inositol trisphosphate, and intracellular Ca2+. The latter effect was blocked by the phospholipase-C inhibitor (PLC) U73122 indicating that Gq proteins mediate GPR75 signaling. RANTES enhanced the phosphorylation of AKT and mitogen-activated protein kinase (MAPK) in GPR75-transfected cells and this effect was blocked by the PLC inhibitor U73122 and the phosphatidylinositol 3-kinase (PI3K) inhibitor, wortmannin. The hippocampal cell line HT22, which expresses GPR75 endogenously, but not the other known RANTES receptors, was used to study the effects of RANTES and GPR75 on neuronal survival. Treatment of HT22 cells with RANTES significantly reduced the neurotoxicity of amyloid-beta peptides, by activating PLC and PI3K.
Conclusions and implications: This demonstrate clearly and undoubtedly the ability of RANTES to act on GPR75. Defects in the RANTES/GPR75-signaling pathway may contribute to neuroinflammatory and neurodegenerative processes as observed in Alzheimer's disease.
Figures


Comment in
-
Tails of the unexpected - an atypical receptor for the chemokine RANTES/CCL5 expressed in brain.Br J Pharmacol. 2006 Nov;149(5):460-2. doi: 10.1038/sj.bjp.0706910. Epub 2006 Sep 25. Br J Pharmacol. 2006. PMID: 17001302 Free PMC article.
References
-
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinl. 2001;22:147–184. - PubMed
-
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. - PubMed
-
- Crane IJ, Kuppner MC, McKillop-Smith S, Knott RM, Forrester JV. Cytokine regulation of RANTES production by human retinal pigment epithelial cells. Cell Immunol. 1998;184:37–44. - PubMed
-
- Cummings JL, Vinters HV, Cole GM, Khachaturian ZS.Alzheimer's disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities Neurology 199851S2–S17.(Discussion S65–S17) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

