Mutagenesis and mechanistic study of a glycoside hydrolase family 54 alpha-L-arabinofuranosidase from Trichoderma koningii
- PMID: 17002602
- PMCID: PMC1820808
- DOI: 10.1042/BJ20060717
Mutagenesis and mechanistic study of a glycoside hydrolase family 54 alpha-L-arabinofuranosidase from Trichoderma koningii
Abstract
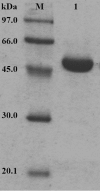
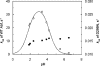
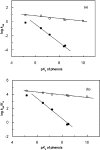
A GH (glycoside hydrolase) family 54 alpha-L-arabinofuranosidase from Trichoderma koningii G-39 (termed Abf) was successfully expressed in Pichia pastoris and purified to near homogeneity by cation-exchange chromatography. To determine the amino acid residues essential for the catalytic activity of Abf, extensive mutagenesis of 24 conserved glutamate and aspartate residues was performed. Among the mutants, D221N, E223Q and D299N were found to decrease catalytic activity significantly. The kcat values of the D221N and D299N mutants were 7000- and 1300-fold lower respectively, than that of the wild-type Abf. E223Q was nearly inactive. These results are consistent with observations obtained from the Aspergillus kawachii alpha-L-arabinofuranosidase three-dimensional structure. This structure indicates that Asp221 of T. koningii Abf is significant for substrate binding and that Glu223 as well as Asp299 function as a nucleophile and a general acid/base catalyst for the enzymatic reaction respectively. The catalytic mechanism of wild-type Abf was further investigated by NMR spectroscopy and kinetic analysis. The results showed that Abf is a retaining enzyme. It catalyses the hydrolysis of various substrates via the formation of a common intermediate that is probably an arabinosyl-enzyme intermediate. A two-step, double-displacement mechanism involving first the formation, and then the breakdown, of an arabinosyl-enzyme intermediate was proposed. Based on the kcat values of a series of aryl-alpha-L-arabinofuranosides catalytically hydrolysed by wild-type Abf, a relatively small Brønsted constant, beta(lg)=-0.18, was obtained, suggesting that the rate-limiting step of the enzymatic reaction is the dearabinosylation step. Further kinetic studies with the D299G mutant revealed that the catalytic activity of this mutant depended largely on the pK(a) values (>6) of leaving phenols, with beta(lg)=-1.3, indicating that the rate-limiting step of the reaction becomes the arabinosylation step. This kinetic outcome supports the idea that Asp299 is the general acid/base residue. The pH activity profile of D299N provided further evidence strengthening this suggestion.
Figures


Similar articles
-
Detailed kinetic analysis and identification of the nucleophile in alpha-L-arabinofuranosidase from Geobacillus stearothermophilus T-6, a family 51 glycoside hydrolase.J Biol Chem. 2002 Nov 15;277(46):43667-73. doi: 10.1074/jbc.M208285200. Epub 2002 Sep 6. J Biol Chem. 2002. PMID: 12221104
-
Mutation of a pH-modulating residue in a GH51 α-l-arabinofuranosidase leads to a severe reduction of the secondary hydrolysis of transfuranosylation products.Biochim Biophys Acta. 2014 Jan;1840(1):626-36. doi: 10.1016/j.bbagen.2013.10.013. Epub 2013 Oct 17. Biochim Biophys Acta. 2014. PMID: 24140392
-
Mutational, kinetic, and NMR studies of the mechanism of E. coli GDP-mannose mannosyl hydrolase, an unusual Nudix enzyme.Biochemistry. 2002 Sep 3;41(35):10834-48. doi: 10.1021/bi020362e. Biochemistry. 2002. PMID: 12196023
-
Function and structure studies of GH family 31 and 97 α-glycosidases.Biosci Biotechnol Biochem. 2011;75(12):2269-77. doi: 10.1271/bbb.110610. Epub 2011 Dec 7. Biosci Biotechnol Biochem. 2011. PMID: 22146713 Review.
-
Mechanistic differences among retaining disaccharide phosphorylases: insights from kinetic analysis of active site mutants of sucrose phosphorylase and alpha,alpha-trehalose phosphorylase.Carbohydr Res. 2008 Aug 11;343(12):2032-40. doi: 10.1016/j.carres.2008.01.029. Epub 2008 Feb 2. Carbohydr Res. 2008. PMID: 18346723 Review.
Cited by
-
An objective criterion to evaluate sequence-similarity networks helps in dividing the protein family sequence space.PLoS Comput Biol. 2023 Aug 16;19(8):e1010881. doi: 10.1371/journal.pcbi.1010881. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37585436 Free PMC article.
-
A Fungal Secretome Adapted for Stress Enabled a Radical Wood Decay Mechanism.mBio. 2021 Aug 31;12(4):e0204021. doi: 10.1128/mBio.02040-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399614 Free PMC article.
-
Insight to Improve α-L-Arabinofuranosidase Productivity in Pichia pastoris and Its Application on Corn Stover Degradation.Front Microbiol. 2018 Dec 14;9:3016. doi: 10.3389/fmicb.2018.03016. eCollection 2018. Front Microbiol. 2018. PMID: 30631307 Free PMC article.
-
Genomics review of holocellulose deconstruction by aspergilli.Microbiol Mol Biol Rev. 2014 Dec;78(4):588-613. doi: 10.1128/MMBR.00019-14. Microbiol Mol Biol Rev. 2014. PMID: 25428936 Free PMC article. Review.
References
-
- Suurnakki A., Tenkanen M., Buchert J., Viikari L. Hemicellulases in the bleaching of chemical pulps. Adv. Biochem. Eng. Biotechnol. 1997;57:261–287. - PubMed
-
- Bezalel L., Shoham Y., Rosenberg E. Characterization and delignification activity of a thermostable α-L-arabinofuranosidase from Bacillus stearothermophilus. Appl. Microbiol. Biotechnol. 1993;40:57–62.
-
- Wong K. K. Y., Sandler J. N. In: Applications of hemicellulases in biopulping, bio-bleaching, food, feed and pulp and paper industries in Hemicellulose and Hemicellulases. Coughlan M. P., Hazlewood G. P., editors. London: Portland Press; 1993. pp. 127–143.
-
- Saha B. C. α-L-Arabinofuranosidases: biochemistry, molecular biology and application in biotechnology. Biotechnol. Adv. 2000;18:403–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

