Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products
- PMID: 17002634
- PMCID: PMC7201869
- DOI: 10.1111/j.1537-2995.2006.00976.x
Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products
Abstract
Background: Severe acute respiratory syndrome coronavirus (SARS-CoV) has been detected in the blood of infected individuals, which may have the potential to contaminate donated blood and plasma-derived products in the event of a future outbreak. Effective methods for inactivating the SARS-CoV in protein solutions are described in this report.
Study design and methods: Heat, ultraviolet (UV) irradiation, octanoic acid, and solvent/detergent (S/D) methods were tested individually for their ability to inactivate SARS-CoV in protein solutions appropriately mimicking blood-derived products. Treated samples were tested for inactivation in a tissue culture growth assay.
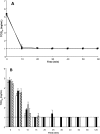
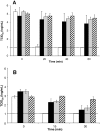
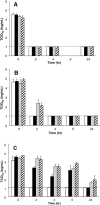
Results: Viral inactivation by heat treatment at 60 degrees C required 15 to 30 minutes to inactivate the SARS-CoV. UVC efficiently inactivated SARS-CoV in 40 minutes, whereas UVA required the addition of psoralen to enhance inactivation of the virus. The presence of bovine serum albumin limited the ability of UVC and UVA to inactivate SARS-CoV and octanoic acid treatment does not reduce the infectivity of SARS-CoV-spiked protein solutions. S/D treatment required 2, 4, and up to 24 hours for Triton X-100, Tween 80, and sodium cholate inactivation, respectively.
Conclusion: Heat, UVC irradiation, and S/D treatments effectively inactivate SARS-CoV, whereas octanoic acid treatment is insufficient for inactivation of the virus.
Figures

References
-
- Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967‐76. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953‐66. - PubMed
-
- Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 2003;300:1394‐9. - PubMed
-
- Duan SM, Zhao XS, Wen RF, et al. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci 2003;16:246‐55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

