Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP
- PMID: 17002803
- PMCID: PMC1592081
- DOI: 10.1186/1744-8069-2-31
Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP
Abstract
Background: The synaptic and cellular mechanisms of pain-related central sensitization in the spinal cord are not fully understood yet. Calcitonin gene-related peptide (CGRP) has been identified as an important molecule in spinal nociceptive processing and ensuing behavioral responses, but its contribution to synaptic plasticity, cellular mechanisms and site of action in the spinal cord remain to be determined. Here we address the role of CGRP in synaptic plasticity in the spinal dorsal horn in a model of arthritic pain.
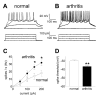
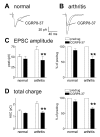
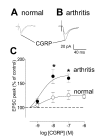
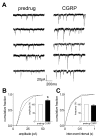
Results: Whole-cell current- and voltage-clamp recordings were made from substantia gelatinosa (SG) neurons in spinal cord slices from control rats and arthritic rats (> 6 h postinjection of kaolin/carrageenan into the knee). Monosynaptic excitatory postsynaptic currents (EPSCs) were evoked by electrical stimulation of afferents in the dorsal root near the dorsal root entry zone. Neurons in slices from arthritic rats showed increased synaptic transmission and excitability compared to controls. A selective CGRP1 receptor antagonist (CGRP8-37) reversed synaptic plasticity in neurons from arthritic rats but had no significant effect on normal transmission. CGRP facilitated synaptic transmission in the arthritis pain model more strongly than under normal conditions where both facilitatory and inhibitory effects were observed. CGRP also increased neuronal excitability. Miniature EPSC analysis suggested a post- rather than pre-synaptic mechanism of CGRP action.
Conclusion: This study is the first to show synaptic plasticity in the spinal dorsal horn in a model of arthritic pain that involves a postsynaptic action of CGRP on SG neurons.
Figures

Similar articles
-
Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior.J Neurosci. 2005 Nov 16;25(46):10717-28. doi: 10.1523/JNEUROSCI.4112-05.2005. J Neurosci. 2005. PMID: 16291945 Free PMC article.
-
Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord.J Neurosci. 2008 May 14;28(20):5189-94. doi: 10.1523/JNEUROSCI.3338-07.2008. J Neurosci. 2008. PMID: 18480275 Free PMC article.
-
Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala.Mol Pain. 2006 May 8;2:18. doi: 10.1186/1744-8069-2-18. Mol Pain. 2006. PMID: 16681859 Free PMC article.
-
Ionotropic glutamate receptors in spinal nociceptive processing.Mol Neurobiol. 2009 Dec;40(3):260-88. doi: 10.1007/s12035-009-8086-8. Epub 2009 Oct 31. Mol Neurobiol. 2009. PMID: 19876771 Review.
-
Calcineurin Regulates Synaptic Plasticity and Nociceptive Transmission at the Spinal Cord Level.Neuroscientist. 2022 Dec;28(6):628-638. doi: 10.1177/10738584211046888. Epub 2021 Nov 18. Neuroscientist. 2022. PMID: 34791930 Review.
Cited by
-
Paralogs of the Calcium-Dependent Activator Protein for Secretion Differentially Regulate Synaptic Transmission and Peptide Secretion in Sensory Neurons.Front Cell Neurosci. 2018 Sep 11;12:304. doi: 10.3389/fncel.2018.00304. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30254567 Free PMC article.
-
Sexual dimorphism in a neuronal mechanism of spinal hyperexcitability across rodent and human models of pathological pain.Brain. 2022 Apr 29;145(3):1124-1138. doi: 10.1093/brain/awab408. Brain. 2022. PMID: 35323848 Free PMC article.
-
Electroacupuncture induced spinal plasticity is linked to multiple gene expressions in dorsal root deafferented rats.J Mol Neurosci. 2009 Feb;37(2):97-110. doi: 10.1007/s12031-008-9095-1. Epub 2008 Jun 26. J Mol Neurosci. 2009. PMID: 18581269
-
Central Role of Protein Kinase A in Promoting Trigeminal Nociception in an In Vivo Model of Temporomandibular Disorders.J Oral Facial Pain Headache. 2017 Summer;31(3):264-274. doi: 10.11607/ofph.1803. J Oral Facial Pain Headache. 2017. PMID: 28738112 Free PMC article.
-
Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice.J Pain. 2009 Sep;10(9):992-1000. doi: 10.1016/j.jpain.2009.03.018. Epub 2009 Jul 22. J Pain. 2009. PMID: 19628434 Free PMC article.
References
-
- Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. 3. New York, Plenum; 2004. pp. 1–962.
-
- Kato G, Yasaka T, Katafuchi T, Furue H, Mizuno M, Iwamoto Y, Yoshimura M. Direct GABAergic and Glycinergic Inhibition of the Substantia Gelatinosa from the Rostral Ventromedial Medulla Revealed by In Vivo Patch-Clamp Analysis in Rats. J Neurosci. 2006;26:1787–1794. doi: 10.1523/JNEUROSCI.4856-05.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

