FLASH is required for histone transcription and S-phase progression
- PMID: 17003125
- PMCID: PMC1578501
- DOI: 10.1073/pnas.0604227103
FLASH is required for histone transcription and S-phase progression
Abstract
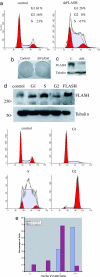
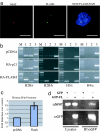
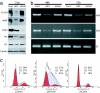
Cajal bodies are nuclear subdomains that are involved in maturation of small ribonucleoproteins and frequently associate with small nuclear RNA and histone gene clusters in interphase cells. We have recently identified FADD-like IL-1beta-converting enzyme (FLICE) associated huge protein (FLASH) as an essential component of Cajal bodies. Here we show that FLASH associates with nuclear protein, ataxia-telangiectasia, a component of the cell-cycle-dependent histone gene transcription machinery. Reduction of FLASH expression by RNA interference results in disruption of the normal Cajal body architecture and relocalization of nuclear protein, ataxia-telangiectasia. Furthermore, FLASH down-regulation results in a clear reduction of histone transcription and a dramatic S-phase arrest of the cell cycle. Chromatin immunoprecipitation reveals that FLASH interacts with histone gene promoter sequences. These results identify FLASH as an important component of the machinery required for histone precursor mRNA expression and cell-cycle progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

