Templated biomineralization on self-assembled protein fibers
- PMID: 17003131
- PMCID: PMC1595410
- DOI: 10.1073/pnas.0602952103
Templated biomineralization on self-assembled protein fibers
Abstract
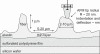
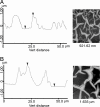
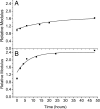
Biological mineralization of tissues in living organisms relies on proteins that preferentially nucleate minerals and control their growth. This process is often referred to as "templating," but this term has become generic, denoting various proposed mineral-organic interactions including both chemical and structural affinities. Here, we present an approach using self-assembled networks of elastin and fibronectin fibers, similar to the extracellular matrix. When induced onto negatively charged sulfonated polystyrene surfaces, these proteins form fiber networks of approximately 10-mum spacing, leaving open regions of disorganized protein between them. We introduce an atomic force microscopy-based technique to measure the elastic modulus of both structured and disorganized protein before and during calcium carbonate mineralization. Mineral-induced thickening and stiffening of the protein fibers during early stages of mineralization is clearly demonstrated, well before discrete mineral crystals are large enough to image by atomic force microscopy. Calcium carbonate stiffens the protein fibers selectively without affecting the regions between them, emphasizing interactions between the mineral and the organized protein fibers. Late-stage observations by optical microscopy and secondary ion mass spectroscopy reveal that Ca is concentrated along the protein fibers and that crystals form preferentially on the fiber crossings. We demonstrate that organized versus unstructured proteins can be assembled mere nanometers apart and probed in identical environments, where mineralization is proved to require the structural organization imposed by fibrillogenesis of the extracellular matrix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

