Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa
- PMID: 17003133
- PMCID: PMC1595434
- DOI: 10.1073/pnas.0602678103
Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa
Abstract
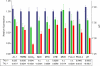
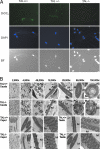
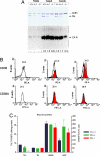
Fertility of spermatozoa depends on maintenance of the mitochondrial transmembrane potential (Deltapsi(m)), which is generated by the electron-transport chain and regulated by an oxidation-reduction equilibrium of reactive oxygen intermediates, pyridine nucleotides, and glutathione (GSH). Here, we report that male mice lacking transaldolase (TAL)(-/-) are sterile because of defective forward motility. TAL(-/-) spermatozoa show loss of Deltapsi(m) and mitochondrial membrane integrity because of diminished NADPH, NADH, and GSH. Mitochondria constitute major Ca(2+) stores; thus, diminished mitochondrial mass accounts for reduced Ca(2+) fluxing, defective forward motility, and infertility. Reduced forward progression of TAL-deficient spermatozoa is associated with diminished mitochondrial reactive oxygen intermediate production and Ca(2+) levels, intracellular acidosis, and compensatory down-regulation of carbonic anhydrase IV and overexpression of CD38 and gamma-glutamyl transferase. Microarray analyses of gene expression in the testis, caput, and cauda epididymidis of TAL(+/+), TAL(+/-), and TAL(-/-) littermates confirmed a dominant impact of TAL deficiency on late stages of sperm-cell development, affecting the electron-transport chain and GSH metabolism. Stimulation of de novo GSH synthesis by oral N-acetyl-cysteine normalized the low fertility rate of TAL(+/-) males without affecting the sterility of TAL(-/-) males. Whereas TAL(-/-) sperm failed to fertilize TAL(+/+) oocytes in vitro, sterility of TAL(-/-) sperm was circumvented by intracytoplasmic sperm injection, indicating that TAL deficiency influenced the structure and function of mitochondria without compromising the nucleus and DNA integrity. Collectively, these data reveal an essential role of TAL in sperm-cell mitochondrial function and, thus, male fertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
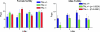

References
-
- Fisher HM, Aitken RJ. J Exp Zool. 1997;277:390–400. - PubMed
-
- Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Hum Reprod. 2002;17:1257–1265. - PubMed
-
- Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Hum Reprod. 2004;19:2267–2276. - PubMed
-
- Perl A, Gergely P, Jr, Puskas F, Banki K. Antiox Redox Signal. 2002;4:427–443. - PubMed
-
- Mayes PA. In: Harper's Biochemistry. Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Norwalk, CT: Appleton & Lange; 1993. pp. 201–211.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

