The role of Pax-6 in lens regeneration
- PMID: 17003134
- PMCID: PMC1595439
- DOI: 10.1073/pnas.0601949103
The role of Pax-6 in lens regeneration
Abstract
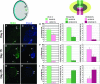
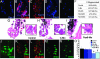
Pax-6 is a master regulator of eye development and is expressed in the dorsal and ventral iris during newt lens regeneration. We show that expression of Pax-6 during newt lens regeneration coincides with cell proliferation. By knocking down expression of Pax-6 via treatment with morpholinos, we found that proliferation of iris pigment epithelial cells was dramatically reduced both in vitro and in vivo, and, as a result, lens regeneration was significantly retarded. However, induction of dedifferentiation in the dorsal iris was not inhibited. Pax-6 knockdown early in lens regeneration resulted in inhibition of crystallin expression and retardation of lens fiber induction. Once crystallin expression and differentiation of lens fibers has ensued, however, loss of function of Pax-6 did not affect crystallin expression and lens fiber maintenance, even though the effects on proliferation persisted. These results conclusively show that Pax-6 is associated with distinct early events during lens regeneration, namely control of cell proliferation and subsequent lens fiber differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Del Rio-Tsonis K, Tsonis PA. Dev Dyn. 2003;226:211–224. - PubMed
-
- Tsonis PA, Del Rio-Tsonis K. Exp Eye Res. 2004;78:161–172. - PubMed
-
- Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K. Int J Dev Biol. 2004;48:975–980. - PubMed
-
- Eguchi G, Shingai R. Dev Growth Differ. 1971;13:337–349. - PubMed
-
- Reyer RW. In: The Visual System in Vertebrates. Crescitelli F, editor. Berlin: Springer; 1977. pp. 309–390.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

