Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG)
- PMID: 17003380
- PMCID: PMC1785141
- DOI: 10.1182/blood-2006-01-024729
Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG)
Abstract
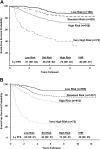
The Children's Cancer Group (CCG) and the Pediatric Oncology Group (POG) joined to form the Children's Oncology Group (COG) in 2000. This merger allowed analysis of clinical, biologic, and early response data predictive of event-free survival (EFS) in acute lymphoblastic leukemia (ALL) to develop a new classification system and treatment algorithm. From 11 779 children (age, 1 to 21.99 years) with newly diagnosed B-precursor ALL consecutively enrolled by the CCG (December 1988 to August 1995, n=4986) and POG (January 1986 to November 1999, n=6793), we retrospectively analyzed 6238 patients (CCG, 1182; POG, 5056) with informative cytogenetic data. Four risk groups were defined as very high risk (VHR; 5-year EFS, 45% or below), lower risk (5-year EFS, at least 85%), and standard and high risk (those remaining in the respective National Cancer Institute [NCI] risk groups). VHR criteria included extreme hypodiploidy (fewer than 44 chromosomes), t(9;22) and/or BCR/ABL, and induction failure. Lower-risk patients were NCI standard risk with either t(12;21) (TEL/AML1) or simultaneous trisomies of chromosomes 4, 10, and 17. Even with treatment differences, there was high concordance between the CCG and POG analyses. The COG risk classification scheme is being used for division of B-precursor ALL into lower- (27%), standard- (32%), high- (37%), and very-high- (4%) risk groups based on age, white blood cell (WBC) count, cytogenetics, day-14 marrow response, and end induction minimal residual disease (MRD) by flow cytometry in COG trials.
Figures

References
-
- Patte C, Auperin A, Michon J, et al. The Societe Francaise d'Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. - PubMed
-
- Winick NJ, Carroll WL, Hunger SP. Childhood leukemia—new advances and challenges. N Engl J Med. 2004;351:601–603. - PubMed
-
- Schrappe M. Evolution of BFM trials for childhood ALL. Ann Hematol. 2004;83(suppl 1):S121–S123. - PubMed
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. - PubMed
-
- Harris MB, Shuster JJ, Carroll A, et al. Trisomy of leukemic cell chromosomes 4 and 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: a Pediatric Oncology Group study. Blood. 1992;79:3316–3324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

