alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis
- PMID: 17003475
- PMCID: PMC1780181
- DOI: 10.2353/ajpath.2006.060058
alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis
Abstract
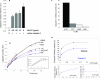
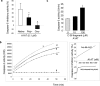
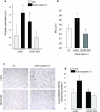
alpha-1 Antitrypsin (A1AT) is an abundant circulating serpin with a postulated function in the lung of potently inhibiting neutrophil-derived proteases. Emphysema attributable to A1AT deficiency led to the concept that a protease/anti-protease imbalance mediates cigarette smoke-induced emphysema. We hypothesized that A1AT has other pathobiological relevant functions in addition to elastase inhibition. We demonstrate a direct prosurvival effect of A1AT through inhibition of lung alveolar endothelial cell apoptosis. Primary pulmonary endothelial cells internalized human A1AT, which co-localized with and inhibited staurosporine-induced caspase-3 activation. In cell-free studies, native A1AT, but not conformers lacking an intact reactive center loop, inhibited the interaction of recombinant active caspase-3 with its specific substrate. Furthermore, overexpression of human A1AT via replication-deficient adeno-associated virus markedly attenuated alveolar wall destruction and oxidative stress caused by caspase-3 instillation in a mouse model of apoptosis-dependent emphysema. Our findings suggest that direct inhibition of active caspase-3 by A1AT may represent a novel anti-apoptotic mechanism relevant to disease processes characterized by excessive structural cell apoptosis, oxidative stress, and inflammation, such as pulmonary emphysema.
Figures




References
-
- Wiedemann HP, Stoller JK. Lung disease due to alpha 1-antitrypsin deficiency. Curr Opin Pulm Med. 1996;2:155–160. - PubMed
-
- The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–185. - PubMed
-
- Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365:2225–2236. - PubMed
-
- Turino GM, Senior RM, Garg BD, Keller S, Levi MM, Mandl I. Serum elastase inhibitor deficiency and alpha 1-antitrypsin deficiency in patients with obstructive emphysema. Science. 1969;165:709–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

