Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes
- PMID: 17003477
- PMCID: PMC1698858
- DOI: 10.2353/ajpath.2006.060434
Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes
Abstract
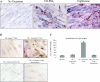
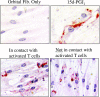
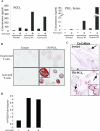
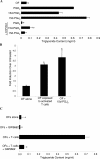
The differentiation of preadipocyte fibroblasts to adipocytes is a crucial process to many disease states including obesity, cardiovascular, and autoimmune diseases. In Graves' disease, the orbit of the eye can become severely inflamed and infiltrated with T lymphocytes as part of the autoimmune process. The orbital fibroblasts convert to fat-like cells causing the eye to protrude, which is disfiguring and can lead to blindness. Recently, the transcription factor peroxisome proliferator activated receptor (PPAR)-gamma and its natural (15d-PGJ2) and synthetic (thiazolidinedione-type) PPAR-gamma agonists have been shown to be crucial to the in vitro differentiation of preadipocyte fibroblasts to adipocytes. We show herein several novel findings. First, that activated T lymphocytes from Graves' patients drive the differentiation of PPAR-gamma-expressing orbital fibroblasts to adipocytes. Second, this adipogenic differentiation is blocked by nonselective small molecule cyclooxygenase (Cox)-1/Cox-2 inhibitors and by Cox-2 selective inhibitors. Third, activated, but not naïve, human T cells highly express Cox-2 and synthesize prostaglandin D2 and related prostaglandins that are PPAR-gamma ligands. These provocative new findings provide evidence for how activated T lymphocytes, through production of PPAR-gamma ligands, profoundly influence human fibroblast differentiation to adipocytes. They also suggest the possibility that, in addition to the orbit, T lymphocytes influence the deposition of fat in other tissues.
Figures




Similar articles
-
Downregulation of cyclooxygenase-2 expression and activation of caspase-3 are involved in peroxisome proliferator-activated receptor-gamma agonists induced apoptosis in human monocyte leukemia cells in vitro.Ann Hematol. 2007 Mar;86(3):173-83. doi: 10.1007/s00277-006-0205-2. Epub 2006 Nov 7. Ann Hematol. 2007. PMID: 17089125
-
Differential modulation of COX-2 expression in A549 airway epithelial cells by structurally distinct PPAR(gamma) agonists: evidence for disparate functional effects which are independent of NF-(kappa)B and PPAR(gamma).Cell Signal. 2005 Sep;17(9):1098-110. doi: 10.1016/j.cellsig.2004.12.002. Epub 2005 Jan 7. Cell Signal. 2005. PMID: 15993751
-
Novel anti-adipogenic activity produced by human fibroblasts.Am J Physiol Cell Physiol. 2010 Sep;299(3):C672-81. doi: 10.1152/ajpcell.00451.2009. Epub 2010 Jun 16. Am J Physiol Cell Physiol. 2010. PMID: 20554910 Free PMC article.
-
Thyroid-associated eye disease.Strabismus. 2000 Jun;8(2):101-11. Strabismus. 2000. PMID: 10980691 Review.
-
PPAR gamma and the control of adipogenesis.Biochimie. 1997 Feb-Mar;79(2-3):111-2. doi: 10.1016/s0300-9084(97)81500-3. Biochimie. 1997. PMID: 9209705 Review.
Cited by
-
Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy.Thyroid. 2008 Sep;18(9):983-8. doi: 10.1089/thy.2007.0404. Thyroid. 2008. PMID: 18788919 Free PMC article. Review.
-
Mechanisms That Underly T Cell Immunity in Graves' Orbitopathy.Front Endocrinol (Lausanne). 2021 Apr 1;12:648732. doi: 10.3389/fendo.2021.648732. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33868176 Free PMC article. Review.
-
The Role of PPARs in Lung Fibrosis.PPAR Res. 2007;2007:71323. doi: 10.1155/2007/71323. PPAR Res. 2007. PMID: 17710235 Free PMC article.
-
Alpha-Linolenic Acid Modulates T Cell Incorporation in a 3D Tissue-Engineered Psoriatic Skin Model.Cells. 2022 Apr 30;11(9):1513. doi: 10.3390/cells11091513. Cells. 2022. PMID: 35563819 Free PMC article.
-
Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy.Invest Ophthalmol Vis Sci. 2014 Mar 20;55(3):1735-48. doi: 10.1167/iovs.14-14002. Invest Ophthalmol Vis Sci. 2014. PMID: 24651704 Free PMC article. Review.
References
-
- Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Prog Clin Biol Res. 1981;54:183–194. - PubMed
-
- Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. - PubMed
-
- Brody JS, Kaplan NB. Proliferation of alveolar interstitial cells during postnatal lung growth. Evidence for two distinct populations of pulmonary fibroblasts. Am Rev Respir Dis. 1983;127:763–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

