Recruitment of a prostaglandin E receptor subtype, EP3-expressing bone marrow cells is crucial in wound-induced angiogenesis
- PMID: 17003499
- PMCID: PMC1780188
- DOI: 10.2353/ajpath.2006.051358
Recruitment of a prostaglandin E receptor subtype, EP3-expressing bone marrow cells is crucial in wound-induced angiogenesis
Abstract
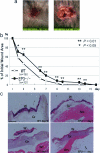
E-type prostaglandins have been reported to be proangiogenic in vivo. Thus, we examined prostaglandin receptor signaling relevant to wound-induced angiogenesis. Full-thickness skin wounds were created on the backs of mice, and angiogenesis in wound granulation tissues was estimated. Wound closure and re-epithelization in EP3 receptor knockout mice (EP3-/-) were significantly delayed compared with their wild-type (WT) mice, whereas those in EP1-/-, EP2-/-, and EP4-/- were not delayed. Wound-induced angiogenesis estimated with CD31 immunohistochemistry in EP3-/- mice was significantly inhibited compared with that in WT mice. Immunoreactive vascular endothelial growth factor (VEGF) in wound granulation tissues in EP3-/- mice was markedly less than that in WT mice. Wound closure in WT mice was delayed significantly by VEGF neutralizing antibody compared with control IgG. Wound-induced angiogenesis and wound closure were significantly suppressed in EP3-/- bone marrow transplantation mice compared with those in WT bone marrow transplantation mice. These were accompanied with the reductions in accumulation of VEGF-expressing cells in wound granulation tissues and in mobilization of VEGF receptor 1-expressing leukocytes in peripheral circulation. These results indicate that the recruitment of EP3-expressing cells to wound granulation tissues is critical for surgical wound healing and angiogenesis via up-regulation of VEGF.
Figures
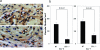



References
-
- Levi S, Goodlad RA, Lee CY, Stamp G, Walport MJ, Wright NA, Hodgson HJ. Inhibitory effect of non-steroidal anti-inflammatory drugs on mucosal cell proliferation associated with gastric ulcer healing. Lancet. 1990;336:840–843. - PubMed
-
- Wang JY, Yamasaki S, Takeuchi K, Okabe S. Delayed healing of acetic acid-induced gastric ulcers in rats by indomethacin. Gastroenterology. 1989;96:393–402. - PubMed
-
- Tarnawski A, Stachura J, Douglass TG, Krause WJ, Gergely H, Sarfeh IJ. Indomethacin impairs quality of experimental gastric ulcer healing: a quality histologic and ultrastructural analysis. Garner A, O’Brien PE, editors. Chichester: J Wiley & Sons,; Mechanisms of Injury, Protection and Repair of the Upper Gastrointestinal Tract. 1991:pp 521–531.
-
- Schmassmann A, Tarnawski A, Peskar BM, Varga L, Flogerzi B, Halter F. Influence of acid and angiogenesis on kinetics of gastric ulcer healing in rats: interaction with indomethacin. Am J Physiol. 1995;268:G276–G285. - PubMed
-
- Tarnawski A. Cellular mechanisms of gastric ulcer healing. Domschke W, Konturek SJ, editors. New York: Springer-Verlag,; The Stomach. 1993:pp 177–192.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

