Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells
- PMID: 17004246
- PMCID: PMC2566533
- DOI: 10.1002/ar.a.20388
Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells
Abstract
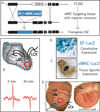
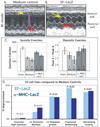
Because pluripotent embryonic stem cells (ESCs) are able to differentiate into any tissue, they are attractive agents for tissue regeneration. Although improvement of cardiac function has been observed after transplantation of pluripotent ESCs, the extent to which these effects reflect ESC-mediated remuscularization, revascularization, or paracrine mechanisms is unknown. Moreover, because ESCs may generate teratomas, the ability to predict the outcome of cellular differentiation, especially when transplanting pluripotent ESCs, is essential; conversely, a requirement to use predifferentiated ESCs would limit their application to highly characterized subsets that are available in limited numbers. In the experiments reported here, we transplanted low numbers of two murine ESC lines, respectively engineered to express a beta-galactosidase gene from either a constitutive (elongation factor) or a cardiac-specific (alpha-myosin heavy chain) promoter, into infarcted mouse myocardium. Although ESC-derived tumors formed within the pericardial space in 21% of injected hearts, lacZ histochemistry revealed that engraftment of ESC was restricted to the ischemic myocardium. Echocardiographic monitoring of ESC-injected hearts that did not form tumors revealed functional improvements by 4 weeks postinfarction, including significant increases in ejection fraction, circumferential fiber shortening velocity, and peak mitral blood flow velocity. These experiments indicate that the infarcted myocardial environment can support engraftment and cardiomyogenic differentiation of pluripotent ESCs, concomitant with partial functional recovery.
Figures

Similar articles
-
Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium.Circulation. 2005 Aug 30;112(9 Suppl):I166-72. doi: 10.1161/CIRCULATIONAHA.104.525824. Circulation. 2005. PMID: 16159810
-
Expandable human cardiovascular progenitors from stem cells for regenerating mouse heart after myocardial infarction.Cardiovasc Res. 2020 Mar 1;116(3):545-553. doi: 10.1093/cvr/cvz181. Cardiovasc Res. 2020. PMID: 31287499 Free PMC article.
-
Embryonic stem cells attenuate myocardial dysfunction and inflammation after surgical global ischemia via paracrine actions.Am J Physiol Heart Circ Physiol. 2008 Oct;295(4):H1726-35. doi: 10.1152/ajpheart.00236.2008. Epub 2008 Aug 22. Am J Physiol Heart Circ Physiol. 2008. PMID: 18723770 Free PMC article.
-
Stem cells in cardiac repair.Future Cardiol. 2011 Jan;7(1):99-117. doi: 10.2217/fca.10.109. Future Cardiol. 2011. PMID: 21174514 Review.
-
Cardiac application of embryonic stem cells.Sheng Li Xue Bao. 2003 Oct 25;55(5):493-504. Sheng Li Xue Bao. 2003. PMID: 14566394 Review.
Cited by
-
Myocardial interstitial fluid inhibits proliferation and cardiomyocyte differentiation in pluripotent embryonic stem cells.Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1369-76. doi: 10.1152/ajpheart.00172.2009. Epub 2009 Jul 24. Am J Physiol Heart Circ Physiol. 2009. PMID: 19633209 Free PMC article.
-
Stem cells for heart cell therapies.Tissue Eng Part B Rev. 2008 Dec;14(4):393-406. doi: 10.1089/ten.teb.2008.0262. Tissue Eng Part B Rev. 2008. PMID: 18821841 Free PMC article.
-
Influence of nanomaterials on stem cell differentiation: designing an appropriate nanobiointerface.Int J Nanomedicine. 2012;7:2211-25. doi: 10.2147/IJN.S29975. Epub 2012 Apr 27. Int J Nanomedicine. 2012. PMID: 22619557 Free PMC article. Review.
-
KCNJ11 knockout morula re-engineered by stem cell diploid aggregation.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):269-76. doi: 10.1098/rstb.2008.0179. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18977736 Free PMC article.
-
Stem cell transplant into preimplantation embryo yields myocardial infarction-resistant adult phenotype.Stem Cells. 2009 Jul;27(7):1697-705. doi: 10.1002/stem.116. Stem Cells. 2009. PMID: 19544428 Free PMC article.
References
-
- Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. Faseb J. 2002;16:1558–1566. - PubMed
-
- Behfar A, Hodgson DM, Zingman LV, Perez-Terzic C, Yamada S, Kane GC, Alekseev AE, Puceat M, Terzic A. Administration of allogenic stem cells dosed to secure cardiogenesis and sustained infarct repair. Ann N Y Acad Sci. 2005;1049:189–198. - PubMed
-
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. - PubMed
-
- Broberg CS, Pantely GA, Barber BJ, Mack GK, Lee K, Thigpen T, Davis LE, Sahn D, Hohimer AR. Validation of the myocardial performance index by echocardiography in mice: a noninvasive measure of left ventricular function. J. Am. Soc. Echocardiogr. 2003;16:814–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

