Prolonged exposure of naïve CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length
- PMID: 17005004
- PMCID: PMC1782358
- DOI: 10.1111/j.1365-2567.2006.02429.x
Prolonged exposure of naïve CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length
Abstract
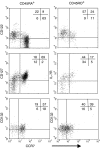
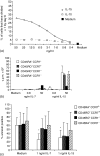
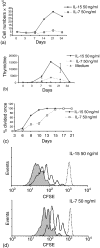
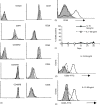
Interleukin (IL)-7 and IL-15 are cytokines implicated in homeostatic control of the peripheral CD8 T-cell pool. We compared the effects of IL-7 and IL-15 on survival and proliferation of purified human CD8+ T-cell subsets. Low concentrations of either cytokine reduced the spontaneous apoptosis of all subsets, and enhancement of survival corresponded to the extent of Bcl-2 up-regulation. Surprisingly, although minimal proliferation of naïve CD8+ T cells was observed during the first week of culture with cytokines, a marked expansion of these cells occurred at later time points, particularly in response to IL-15. This occurred largely without phenotypic change or acquisition of effector function, indicating a dissociation of differentiation from proliferation. Notably, progression of naïve CD8+ T cells through several cell divisions resulted in up-regulation of telomerase and the maintenance of telomere length. These data show that IL-7 and IL-15 induce cell proliferation and rescue from apoptosis in a concentration, time and subset-dependent manner, and have implications for the homeostatic expansion of the naïve CD8+ T-cell pool.
Figures


Similar articles
-
IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire.J Immunol. 1998 Dec 1;161(11):5909-17. J Immunol. 1998. PMID: 9834071
-
Cytokine deprivation of naive CD8+ T cells promotes minimal cell cycling but maximal cytokine synthesis and autonomous proliferation subsequently: a mechanism of self-regulation.J Immunol. 1999 Sep 1;163(5):2443-51. J Immunol. 1999. PMID: 10452979
-
IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL.Eur J Immunol. 2007 Jun;37(6):1678-90. doi: 10.1002/eji.200636329. Eur J Immunol. 2007. PMID: 17492620
-
Cytokine control of memory T-cell development and survival.Nat Rev Immunol. 2003 Apr;3(4):269-79. doi: 10.1038/nri1052. Nat Rev Immunol. 2003. PMID: 12669018 Review.
-
Homeostasis of memory T cells.Immunol Rev. 2006 Jun;211:154-63. doi: 10.1111/j.0105-2896.2006.00401.x. Immunol Rev. 2006. PMID: 16824125 Review.
Cited by
-
Improving the ex vivo expansion of human tumor-reactive CD8 + T cells by targeting toll-like receptors.Front Bioeng Biotechnol. 2022 Oct 31;10:1027619. doi: 10.3389/fbioe.2022.1027619. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36394017 Free PMC article.
-
Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+CD45RA+CD27+ T cells: the potential involvement of interleukin-7 in this process.Immunology. 2011 Mar;132(3):326-39. doi: 10.1111/j.1365-2567.2010.03386.x. Epub 2011 Jan 7. Immunology. 2011. PMID: 21214539 Free PMC article.
-
Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity?Nat Rev Immunol. 2019 Sep;19(9):563-572. doi: 10.1038/s41577-019-0177-9. Nat Rev Immunol. 2019. PMID: 31175337 Review.
-
Synthetic cytokine circuits that drive T cells into immune-excluded tumors.Science. 2022 Dec 16;378(6625):eaba1624. doi: 10.1126/science.aba1624. Epub 2022 Dec 16. Science. 2022. PMID: 36520915 Free PMC article.
-
Telomeres and immunological diseases of aging.Gerontology. 2010;56(4):390-403. doi: 10.1159/000268620. Epub 2009 Dec 17. Gerontology. 2010. PMID: 20016137 Free PMC article. Review.
References
-
- Beverley PC. Kinetics and clonality of immunological memory in humans. Semin Immunol. 2004;16:315–21. - PubMed
-
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. - PubMed
-
- Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De Bruyere M, Strauss K. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70:152–8. - PubMed
-
- Macallan DC, Asquith B, Irvine AJ, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

