Soluble and insoluble signals and the induction of bone formation: molecular therapeutics recapitulating development
- PMID: 17005018
- PMCID: PMC2100361
- DOI: 10.1111/j.1469-7580.2006.00635.x
Soluble and insoluble signals and the induction of bone formation: molecular therapeutics recapitulating development
Abstract
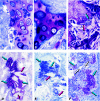
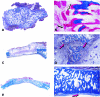
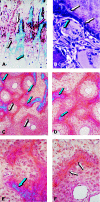
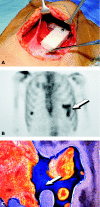
The osteogenic molecular signals of the transforming growth factor-beta (TGF-beta) superfamily, the bone morphogenetic/osteogenic proteins (BMPs/OPs) and uniquely in primates the TGF-beta isoforms per se, pleiotropic members of the TGF-beta supergene family, induce de novo endochondral bone formation as a recapitulation of embryonic development. Naturally derived BMPs/OPs and gamma-irradiated human recombinant osteogenic protein-1 (hOP-1) delivered by allogeneic and xenogeneic insoluble collagenous matrices initiate de novo bone induction in heterotopic and orthotopic sites of the primate Papio ursinus, culminating in complete calvarial regeneration by day 90 and maintaining the regenerated structures by day 365. The induction of bone by hOP-1 in P. ursinus develops as a mosaic structure with distinct spatial and temporal patterns of gene expression of members of the TGF-beta superfamily that singly, synergistically and synchronously initiate and maintain tissue induction and morphogenesis. The temporal and spatial expressions of TGF-beta1 mRNA indicate a specific temporal transcriptional window during which expression of TGF-beta1 is mandatory for successful and optimal osteogenesis. Highly purified naturally derived bovine BMPs/OPs and hOP-1 delivered by human collagenous bone matrices and porous hydroxyapatite, respectively, induce bone formation in mandibular defects of human patients. By using healthy body sites as bioreactors it is possible to recapitulate embryonic developments by inducing selected biomaterials combined with recombinant proteins to transform into custom-made prefabricated bone grafts for human reconstruction. The osteogenic proteins of the TGF-beta superfamily, BMPs/OPs and TGF-betas, the last endowed with the striking prerogative of inducing endochondral bone formation in primates only, are helping to engineer skeletal reconstruction in molecular terms.
Figures






References
-
- Åberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–396. - PubMed
-
- Bertelsen A. Experimental investigations into post-foetal osteogenesis. Acta Orthop Scand. 1945;15:139–181.
-
- Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005;63:1693–1707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

