Location of injury influences the mechanisms of both regeneration and repair within the MRL/MpJ mouse
- PMID: 17005026
- PMCID: PMC2100365
- DOI: 10.1111/j.1469-7580.2006.00641.x
Location of injury influences the mechanisms of both regeneration and repair within the MRL/MpJ mouse
Abstract
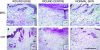
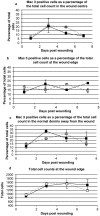
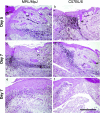
The adult MRL/MpJ mouse regenerates all differentiated structures after through-and-through ear punch wounding in a scar-free process. We investigated whether this regenerative capacity was also shown by skin wounds. Dorsal skin wounds were created, harvested and archived from the same animals (MRL/MpJ and C57BL/6 mice) that received through-and-through ear punch wounds. Re-epithelialization was complete in dorsal wounds in both strains by day 5 and extensive granulation tissue was present by day 14 post-wounding. By day 21, wounds from both strains contained dense amounts of collagen that healed with a scar. The average wound area, as well as alpha-smooth muscle actin expression and macrophage influx were investigated during dorsal skin wound healing and did not significantly differ between strains. Thus, MRL/MpJ mice regenerate ear wounds in a scar-free manner, but heal dorsal skin wounds by simple repair with scar formation. A significant conclusion can be drawn from these data; mechanisms of regeneration and repair can occur within the same animal, potentially utilizing similar molecules and signalling pathways that subtly diverge dependent upon the microenvironment of the injury.
Figures
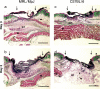
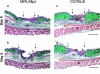
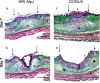
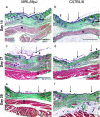

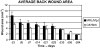
Similar articles
-
Skin wounds in the MRL/MPJ mouse heal with scar.Wound Repair Regen. 2006 Jan-Feb;14(1):81-90. doi: 10.1111/j.1524-475X.2005.00092.x. Wound Repair Regen. 2006. PMID: 16476076
-
Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma.Dev Dyn. 2003 Feb;226(2):388-97. doi: 10.1002/dvdy.10242. Dev Dyn. 2003. PMID: 12557217
-
Differential cutaneous wound healing in thermally injured MRL/MPJ mice.Wound Repair Regen. 2007 Jul-Aug;15(4):577-88. doi: 10.1111/j.1524-475X.2007.00266.x. Wound Repair Regen. 2007. PMID: 17650103
-
Characterizing regeneration in the vertebrate ear.J Anat. 2006 Oct;209(4):439-46. doi: 10.1111/j.1469-7580.2006.00632.x. J Anat. 2006. PMID: 17005017 Free PMC article. Review.
-
Scar-free healing: from embryonic mechanisms to adult therapeutic intervention.Philos Trans R Soc Lond B Biol Sci. 2004 May 29;359(1445):839-50. doi: 10.1098/rstb.2004.1475. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15293811 Free PMC article. Review.
Cited by
-
Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates.PLoS One. 2012;7(4):e32875. doi: 10.1371/journal.pone.0032875. Epub 2012 Apr 2. PLoS One. 2012. PMID: 22485136 Free PMC article.
-
Improved biomechanical and biological outcomes in the MRL/MpJ murine strain following a full-length patellar tendon injury.J Orthop Res. 2015 Nov;33(11):1693-703. doi: 10.1002/jor.22928. Epub 2015 May 25. J Orthop Res. 2015. PMID: 25982892 Free PMC article.
-
The super super-healing MRL mouse strain.Front Biol (Beijing). 2012 Dec 1;7(6):522-538. doi: 10.1007/s11515-012-1192-4. Front Biol (Beijing). 2012. PMID: 24163690 Free PMC article.
-
Macrophages are necessary for epimorphic regeneration in African spiny mice.Elife. 2017 May 16;6:e24623. doi: 10.7554/eLife.24623. Elife. 2017. PMID: 28508748 Free PMC article.
-
Keratin gene expression profiles after digit amputation in C57BL/6 vs. regenerative MRL mice imply an early regenerative keratinocyte activated-like state.Physiol Genomics. 2013 Jun 3;45(11):409-21. doi: 10.1152/physiolgenomics.00142.2012. Epub 2013 Mar 19. Physiol Genomics. 2013. PMID: 23512742 Free PMC article.
References
-
- Bai S, Thummel R, Godwin AR, et al. Matrix metalloproteinase expression and function during fin regeneration in zebrafish: analysis of MT1-MMP, MMP2 and TIMP2. Matrix Biol. 2005;24:247–260. - PubMed
-
- Beare AH, O'Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–163. - PubMed
-
- Christensen RN, Weinstein M, Tassava RA. Expression of fibroblast growth factors 4, 8, and 10 in limbs, flanks, and blastemas of Ambystoma. Dev Dyn. 2002;223:193–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

