Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD
- PMID: 17005052
- PMCID: PMC1609108
- DOI: 10.1186/1742-2094-3-27
Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD
Abstract
Background: Microglia are associated with neuritic plaques in Alzheimer disease (AD) and serve as a primary component of the innate immune response in the brain. Neuritic plaques are fibrous deposits composed of the amyloid beta-peptide fragments (Abeta) of the amyloid precursor protein (APP). Numerous studies have shown that the immune cells in the vicinity of amyloid deposits in AD express mRNA and proteins for pro-inflammatory cytokines, leading to the hypothesis that microglia demonstrate classical (Th-1) immune activation in AD. Nonetheless, the complex role of microglial activation has yet to be fully explored since recent studies show that peripheral macrophages enter an "alternative" activation state.
Methods: To study alternative activation of microglia, we used quantitative RT-PCR to identify genes associated with alternative activation in microglia, including arginase I (AGI), mannose receptor (MRC1), found in inflammatory zone 1 (FIZZ1), and chitinase 3-like 3 (YM1).
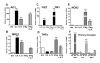
Results: Our findings confirmed that treatment of microglia with anti-inflammatory cytokines such as IL-4 and IL-13 induces a gene profile typical of alternative activation similar to that previously observed in peripheral macrophages. We then used this gene expression profile to examine two mouse models of AD, the APPsw (Tg-2576) and Tg-SwDI, models for amyloid deposition and for cerebral amyloid angiopathy (CAA) respectively. AGI, MRC1 and YM1 mRNA levels were significantly increased in the Tg-2576 mouse brains compared to age-matched controls while TNFalpha and NOS2 mRNA levels, genes commonly associated with classical activation, increased or did not change, respectively. Only TNFalpha mRNA increased in the Tg-SwDI mouse brain. Alternative activation genes were also identified in brain samples from individuals with AD and were compared to age-matched control individuals. In AD brain, mRNAs for TNFalpha, AGI, MRC1 and the chitinase-3 like 1 and 2 genes (CHI3L1; CHI3L2) were significantly increased while NOS2 and IL-1beta mRNAs were unchanged.
Conclusion: Immune cells within the brain display gene profiles that suggest heterogeneous, functional phenotypes that range from a pro-inflammatory, classical activation state to an alternative activation state involved in repair and extracellular matrix remodeling. Our data suggest that innate immune cells in AD may exhibit a hybrid activation state that includes characteristics of classical and alternative activation.
Figures
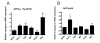

References
-
- Hume D. The mononuclear phagocyte system revisited. J Leukocyte Biol. 2004;72:621–627. - PubMed
-
- Adams D. Regulation of macrophage function by interferon-γ. In: Baron S, Coppenhaver D, Dianzani F, Fleischmann, W, Hughes T, Klimpel G, Niesel D, Stanton G, Tyring S, editor. Interferon. Galveston, TX: University of Texas Medical Branch; 1992. pp. 341–351.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

