Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex
- PMID: 17005651
- PMCID: PMC1676300
- DOI: 10.1128/JVI.01565-06
Role of ran binding protein 5 in nuclear import and assembly of the influenza virus RNA polymerase complex
Abstract
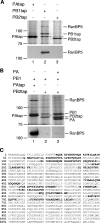
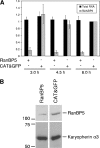
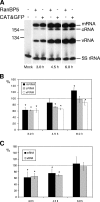
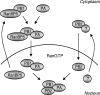
The influenza A virus RNA-dependent RNA polymerase is a heterotrimeric complex of polymerase basic protein 1 (PB1), PB2, and polymerase acidic protein (PA) subunits. It performs transcription and replication of the viral RNA genome in the nucleus of infected cells. We have identified a nuclear import factor, Ran binding protein 5 (RanBP5), also known as karyopherin beta3, importin beta3, or importin 5, as an interactor of the PB1 subunit. RanBP5 interacted with either PB1 alone or with a PB1-PA dimer but not with a PB1-PB2 dimer or the trimeric complex. The interaction between RanBP5 and PB1-PA was disrupted by RanGTP in vitro, allowing PB2 to bind to the PB1-PA dimer to form a functional trimeric RNA polymerase complex. We propose a model in which RanBP5 acts as an import factor for the newly synthesized polymerase by targeting the PB1-PA dimer to the nucleus. In agreement with this model, small interfering RNA (siRNA)-mediated knock-down of RanBP5 inhibited the nuclear accumulation of the PB1-PA dimer. Moreover, siRNA knock-down of RanBP5 resulted in the delayed accumulation of viral RNAs in infected cells, confirming that RanBP5 plays a biological role during the influenza virus life cycle.
Figures
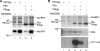

References
-
- Bayliss, R., A. H. Corbett, and M. Stewart. 2000. The molecular mechanism of transport of macromolecules through nuclear pore complexes. Traffic 1:448-456. - PubMed
-
- Bullido, R., P. Gómez-Puertas, C. Albo, and A. Portela. 2000. Several protein regions contribute to determine the nuclear and cytoplasmic localization of the influenza A virus nucleoprotein. J. Gen. Virol. 81:135-142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

