Divergences in protein activity and cellular localization within the Campoletis sonorensis Ichnovirus Vankyrin family
- PMID: 17005654
- PMCID: PMC1676293
- DOI: 10.1128/JVI.01187-06
Divergences in protein activity and cellular localization within the Campoletis sonorensis Ichnovirus Vankyrin family
Abstract
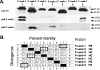
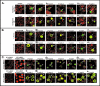
Ichnoviruses (IVs) occur in obligate symbiotic associations with endoparasitic ichneumonid wasps. IVs are injected with eggs during parasitization, where viral infection and gene expression alter host physiology to ensure endoparasitoid survival. The seven Campoletis sonorensis IV (CsIV) vankyrin genes encode proteins that possess ankyrin repeat domains resembling the inhibitory domains of NF-kappaB transcription factor inhibitors (IkappaBs). The CsIV vankyrins are divided into two subclasses: those expressed primarily in the host fat body (three genes) and those expressed in host hemocytes (four genes). CsIV vankyrin proteins showed limited antigenic similarity when analyzed by Western blotting. Cellular localization and expression patterns of recombinant vankyrin proteins in High Five and Sf9 insect cells differed within and between the subclasses and in cells exposed to lipopolysaccharide, laminarin, or viral immune challenge. In unstimulated Sf9 cells, five vankyrins were detected in cell nuclei. The remaining two proteins localized predominantly to cytoplasmic granules. Immune stimulation of cells resulted in a nuclear-to-cytoplasmic shift of three vankyrins but did not affect localization of other variants. When expressed from recombinant Autographa californica multiple nucleopolyhedroviruses (AcMNPVs), all vankyrins showed a nuclear localization during early stages of infection with patterns resembling those of immune-challenged cells as the infection progressed. Two fat body vankyrins also produced unique biological effects when expressed from recombinant AcMNPV. Insect cells infected with these viruses exhibited enhanced longevity compared to those infected with viruses expressing other vankyrins. Together, these data suggest that vankyrin proteins in CsIV have divergent physiological functions.
Figures



Similar articles
-
Ikappabeta-related vankyrin genes in the Campoletis sonorensis ichnovirus: temporal and tissue-specific patterns of expression in parasitized Heliothis virescens lepidopteran hosts.J Virol. 2005 Jun;79(12):7617-28. doi: 10.1128/JVI.79.12.7617-7628.2005. J Virol. 2005. PMID: 15919914 Free PMC article.
-
Cloning and characterization of two Campoletis chlorideae ichnovirus vankyrin genes expressed in parasitized host Helicoverpa armigera.J Insect Physiol. 2007 Jul;53(7):699-707. doi: 10.1016/j.jinsphys.2007.03.015. Epub 2007 Apr 10. J Insect Physiol. 2007. PMID: 17512002
-
The Campoletis sonorensis ichnovirus vankyrin protein P-vank-1 inhibits apoptosis in insect Sf9 cells.Insect Mol Biol. 2009 Aug;18(4):497-506. doi: 10.1111/j.1365-2583.2009.00892.x. Epub 2009 May 15. Insect Mol Biol. 2009. PMID: 19453763
-
Origin and evolution of polydnaviruses by symbiogenesis of insect DNA viruses in endoparasitic wasps.J Insect Physiol. 2003 May;49(5):419-32. doi: 10.1016/s0022-1910(03)00059-3. J Insect Physiol. 2003. PMID: 12770621 Review.
-
Unfolding the evolutionary story of polydnaviruses.Virus Res. 2006 Apr;117(1):81-9. doi: 10.1016/j.virusres.2006.01.001. Epub 2006 Feb 3. Virus Res. 2006. PMID: 16460826 Review.
Cited by
-
Improving the baculovirus expression vector system with vankyrin-enhanced technology.Biotechnol Prog. 2017 Nov;33(6):1496-1507. doi: 10.1002/btpr.2516. Epub 2017 Jul 6. Biotechnol Prog. 2017. PMID: 28649776 Free PMC article.
-
Multigenic families in Ichnovirus: a tissue and host specificity study through expression analysis of vankyrins from Hyposoter didymator Ichnovirus.PLoS One. 2011;6(11):e27522. doi: 10.1371/journal.pone.0027522. Epub 2011 Nov 8. PLoS One. 2011. PMID: 22087334 Free PMC article.
-
Extensive transcription analysis of the Hyposoter didymator Ichnovirus genome in permissive and non-permissive lepidopteran host species.PLoS One. 2014 Aug 12;9(8):e104072. doi: 10.1371/journal.pone.0104072. eCollection 2014. PLoS One. 2014. PMID: 25117496 Free PMC article.
-
A new gene family (BAPs) of Cotesia bracovirus induces apoptosis of host hemocytes.Virulence. 2023 Dec;14(1):2171691. doi: 10.1080/21505594.2023.2171691. Virulence. 2023. PMID: 36694288 Free PMC article.
-
Identification of an in vitro interaction between an insect immune suppressor protein (CrV2) and G alpha proteins.J Biol Chem. 2011 Mar 25;286(12):10466-75. doi: 10.1074/jbc.M110.214726. Epub 2011 Jan 13. J Biol Chem. 2011. PMID: 21233205 Free PMC article.
References
-
- Asgari, S., and O. Schmidt. 2002. A coiled-coil region of an insect immune suppressor protein is involved in binding and uptake by hemocytes. Insect Biochem. Mol. Biol. 32:497-504. - PubMed
-
- Beck, M., and M. R. Strand. 2003. RNA interference silences Microplitis demolitor bracovirus genes and implicates glc1.8 in disruption of adhesion in infected host cells. Virology 314:521-535. - PubMed
-
- Beckage, N. E. 1998. Modulation of immune responses to parasitoids by polydnaviruses. Parasitology 116(Suppl.):S57-S64. - PubMed
-
- Beg, A. A., and A. S. Baldwin, Jr. 1993. The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 7:2064-2070. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

