In vivo packaging of brome mosaic virus RNA3, but not RNAs 1 and 2, is dependent on a cis-acting 3' tRNA-like structure
- PMID: 17005656
- PMCID: PMC1797238
- DOI: 10.1128/JVI.01500-06
In vivo packaging of brome mosaic virus RNA3, but not RNAs 1 and 2, is dependent on a cis-acting 3' tRNA-like structure
Abstract
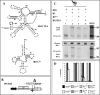
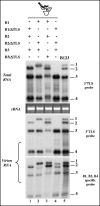
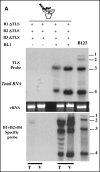
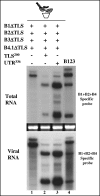
The four encapsidated RNAs of brome mosaic virus (BMV; B1, B2, B3, and B4) contain a highly conserved 3' 200-nucleotide (nt) region encompassing the tRNA-like structure (TLS) which is required for packaging in vitro (Y. G. Choi, T. W. Dreher, and A. L. N. Rao, Proc. Natl. Acad. Sci. USA 99:655-660, 2002). To validate these observations in vivo, we performed packaging assays using Agrobacterium-mediated transient expression of RNAs and coat protein (CP) (P. Annamalai and A. L. N. Rao, Virology 338:96-111, 2005). Coexpression of TLS-less constructs of B1 or B2 or B3 and CP mRNAs in Nicotiana benthamiana leaves resulted in packaging of TLS-less B1 and B2 but not B3, suggesting that packaging of B3 requires the TLS in cis. This conjecture was confirmed by the efficient packaging of a B3 chimera in which the viral TLS was replaced with a cellular tRNA(Tyr). When N. benthamiana leaves were infiltrated with a mixture of transformants containing wild-type B1 (wtB1) plus wtB2 plus a TLS-less B3 (wtB1+wtB2+TLS-lessB3), the 3' end of progeny B3 was restored by heterologous recombination with that of either B1 or B2. This intrinsic cis-requirement of TLS in promoting B3 packaging was further confirmed when a mixture containing agrotransformants of TLS-less B1+B2+B3 was supplemented with either wtB4 or a 3' 200-nt or 3' 336-nt untranslated region (UTR) of B3. Northern blot analysis followed by sequencing of B3 progeny revealed that replication of TLS-less B3, but not TLS-less B1 or B2, was fully restored due to recombination with TLS from transiently expressed wtB4 or the B3 3' UTR. Collectively, these observations suggested that the requirement of a cis-acting TLS is distinct for B3 compared with B1 or B2.
Figures

References
-
- Annamalai, P., and A. L. Rao. 2006. Delivery and Expression of functional viral RNA genomes in planta by agroinfiltration, p. 16B.2.1-2.15. In T. Downey (ed.), Current protocols in microbiology, vol. 1. John Wiley & Sons, Inc., Hoboken, NJ. - PubMed
-
- Annamalai, P., and A. L. Rao. 2005. Dispensability of 3′ tRNA-like sequence for packaging cowpea chlorotic mottle virus genomic RNAs. Virology 332:650-658. - PubMed
-
- Annamalai, P., and A. L. Rao. 2005. Replication-independent expression of genome components and capsid protein of brome mosaic virus in planta: a functional role for viral replicase in RNA packaging. Virology 338:96-111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

