Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells
- PMID: 17005662
- PMCID: PMC1676308
- DOI: 10.1128/JVI.00890-06
Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells
Abstract
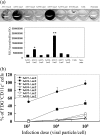
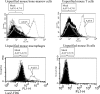
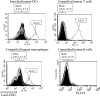
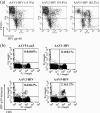
The ability of adeno-associated virus serotype 1 to 8 (AAV1 to AAV8) vectors expressing the human immunodeficiency virus type 1 (HIV-1) Env gp160 (AAV-HIV) to induce an immune response was evaluated in BALB/c mice. The AAV5 vector showed a higher tropism for both mouse and human dendritic cells (DCs) than did the AAV2 vector, whereas other AAV serotype vectors transduced DCs only poorly. AAV1, AAV5, AAV7, and AAV8 were more highly expressed in muscle cells than AAV2. An immunogenicity study of AAV serotypes indicates that AAV1, AAV5, AAV7, and AAV8 vectors expressing the Env gp160 gene induced higher HIV-specific humoral and cell-mediated immune responses than the AAV2 vector did, with the AAV5 vector producing the best responses. Furthermore, mice injected with DCs that had been transduced ex vivo with an AAV5 vector expressing the gp160 gene elicited higher HIV-specific cell-mediated immune responses than did DCs transduced with AAV1 and AAV2 vectors. We also found that AAV vectors produced by HEK293 cells and insect cells elicit similar levels of antigen-specific immune responses. These results demonstrate that the immunogenicity of AAV vectors depends on their tropism for both antigen-presenting cells (such as DCs) and non-antigen-presenting cells (such as muscular cells) and that AAV5 is a better vector than other AAV serotypes. These results may aid in the development of AAV-based vaccine and gene therapy.
Figures




Similar articles
-
Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: implications for gene therapy using AAV vectors.Gene Ther. 2014 Aug;21(8):732-8. doi: 10.1038/gt.2014.47. Epub 2014 May 22. Gene Ther. 2014. PMID: 24849042
-
Pre-existing antibodies to candidate gene therapy vectors (adeno-associated vector serotypes) in domestic cats.PLoS One. 2019 Mar 21;14(3):e0212811. doi: 10.1371/journal.pone.0212811. eCollection 2019. PLoS One. 2019. PMID: 30897117 Free PMC article.
-
Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain.Neuroscience. 2006;138(2):501-10. doi: 10.1016/j.neuroscience.2005.11.057. Epub 2006 Jan 18. Neuroscience. 2006. PMID: 16414198
-
Immune responses to adeno-associated virus vectors.Curr Gene Ther. 2005 Jun;5(3):323-31. doi: 10.2174/1566523054065039. Curr Gene Ther. 2005. PMID: 15975009 Review.
-
Adeno-associated virus (AAV) vectors in gene therapy: immune challenges and strategies to circumvent them.Rev Med Virol. 2013 Nov;23(6):399-413. doi: 10.1002/rmv.1762. Epub 2013 Sep 10. Rev Med Virol. 2013. PMID: 24023004 Review.
Cited by
-
Major subsets of human dendritic cells are efficiently transduced by self-complementary adeno-associated virus vectors 1 and 2.J Virol. 2007 May;81(10):5385-94. doi: 10.1128/JVI.02516-06. Epub 2007 Feb 21. J Virol. 2007. PMID: 17314166 Free PMC article.
-
HIV-1 gp120-induced migration of dendritic cells is regulated by a novel kinase cascade involving Pyk2, p38 MAP kinase, and LSP1.Blood. 2009 Oct 22;114(17):3588-600. doi: 10.1182/blood-2009-02-206342. Epub 2009 Aug 21. Blood. 2009. PMID: 19700666 Free PMC article.
-
Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery.Pharmacol Ther. 2020 Mar;207:107453. doi: 10.1016/j.pharmthera.2019.107453. Epub 2019 Dec 11. Pharmacol Ther. 2020. PMID: 31836454 Free PMC article. Review.
-
Duchenne muscular dystrophy gene therapy in the canine model.Hum Gene Ther Clin Dev. 2015 Mar;26(1):57-69. doi: 10.1089/humc.2015.006. Epub 2015 Feb 24. Hum Gene Ther Clin Dev. 2015. PMID: 25710459 Free PMC article. Review.
-
Strategies to modulate immune responses: a new frontier for gene therapy.Mol Ther. 2009 Sep;17(9):1492-503. doi: 10.1038/mt.2009.150. Epub 2009 Jul 7. Mol Ther. 2009. PMID: 19584819 Free PMC article. Review.
References
-
- Aldrich, W. A., C. Ren, A. F. White, S. Z. Zhou, S. Kumar, C. B. Jenkins, D. R. Shaw, T. V. Strong, P. L. Triozzi, and S. Ponnazhagan. 2006. Enhanced transduction of mouse bone marrow-derived dendritic cells by repetitive infection with self-complementary adeno-associated virus 6 combined with immunostimulatory ligands. Gene Ther. 13:29-39. - PubMed
-
- Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Babcock, A. M., D. Standing, K. Bullshields, E. Schwartz, C. M. Paden, and D. J. Poulsen. 2005. In vivo inhibition of hippocampal Ca2+/calmodulin-dependent protein kinase II by RNA interference. Mol. Ther. 11:899-905. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

