Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome
- PMID: 17005665
- PMCID: PMC1642595
- DOI: 10.1128/JVI.00971-06
Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome
Abstract
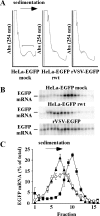
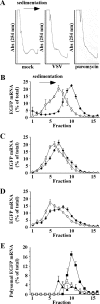
Host protein synthesis is inhibited in cells infected with vesicular stomatitis virus (VSV). It has been proposed that viral mRNAs are subjected to the same inhibition but are predominantly translated because of their abundance. To compare translation efficiencies of viral and host mRNAs during infection, we used an enhanced green fluorescent protein (EGFP) reporter expressed from a recombinant virus or from the host nucleus in stably transfected cells. Translation efficiency of host-derived EGFP mRNA was reduced more than threefold at eight hours postinfection, while viral-derived mRNA was translated around sevenfold more efficiently than host-derived EGFP mRNA in VSV-infected cells. To test whether mRNAs transcribed in the cytoplasm are resistant to shutoff of translation during VSV infection, HeLa cells were infected with a recombinant simian virus 5 (rSV5) that expressed GFP. Cells were then superinfected with VSV or mock superinfected. GFP mRNA transcribed by rSV5 was not resistant to translation inhibition during superinfection with VSV, indicating that transcription in the cytoplasm is not sufficient for preventing translation inhibition. To determine if cis-acting sequences in untranslated regions (UTRs) were involved in preferential translation of VSV mRNAs, we constructed EGFP reporters with VSV or control UTRs and measured the translation efficiency in mock-infected and VSV-infected cells. The presence of VSV UTRs did not affect mRNA translation efficiency in mock- or VSV-infected cells, indicating that VSV mRNAs do not contain cis-acting sequences that influence translation. However, we found that when EGFP mRNAs transcribed by VSV or by the host were translated in vitro, VSV-derived EGFP mRNA was translated 22 times more efficiently than host-derived EGFP mRNA. This indicated that VSV mRNAs do contain cis-acting structural elements (that are not sequence based), which enhance translation efficiency of viral mRNAs.
Figures




Similar articles
-
New mRNAs are preferentially translated during vesicular stomatitis virus infection.J Virol. 2008 Mar;82(5):2286-94. doi: 10.1128/JVI.01761-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094194 Free PMC article.
-
Vesicular stomatitis virus mRNA and inhibition of translation of cellular mRNA--is there a P function in vesicular stomatitis virus?J Virol. 1981 May;38(2):504-17. doi: 10.1128/JVI.38.2.504-517.1981. J Virol. 1981. PMID: 6264124 Free PMC article.
-
the influence of the host cell on the inhibition of virus protein synthesis in cells double infected with vesicular stomatitis virus and mengovirus.J Gen Virol. 1980 Oct;50(2):293-307. doi: 10.1099/0022-1317-50-2-293. J Gen Virol. 1980. PMID: 6257824
-
Inhibition of cell functions by RNA-virus infections.Annu Rev Microbiol. 1984;38:91-109. doi: 10.1146/annurev.mi.38.100184.000515. Annu Rev Microbiol. 1984. PMID: 6093688 Review.
-
Vesicular stomatitis virus: mode of transcription.J Gen Virol. 1977 Jan;34(1):1-8. doi: 10.1099/0022-1317-34-1-1. J Gen Virol. 1977. PMID: 188975 Review.
Cited by
-
The VSV matrix protein inhibits NF-κB and the interferon response independently in mouse L929 cells.Virology. 2020 Sep;548:117-123. doi: 10.1016/j.virol.2020.06.013. Epub 2020 Jun 29. Virology. 2020. PMID: 32838932 Free PMC article.
-
Global analysis of polysome-associated mRNA in vesicular stomatitis virus infected cells.PLoS Pathog. 2019 Jun 21;15(6):e1007875. doi: 10.1371/journal.ppat.1007875. eCollection 2019 Jun. PLoS Pathog. 2019. PMID: 31226162 Free PMC article.
-
Applications of Replicating-Competent Reporter-Expressing Viruses in Diagnostic and Molecular Virology.Viruses. 2016 May 6;8(5):127. doi: 10.3390/v8050127. Viruses. 2016. PMID: 27164126 Free PMC article. Review.
-
Lack of Ikaros Deregulates Inflammatory Gene Programs in T Cells.J Immunol. 2019 Feb 15;202(4):1112-1123. doi: 10.4049/jimmunol.1801270. Epub 2019 Jan 11. J Immunol. 2019. PMID: 30635395 Free PMC article.
-
The Egyptian Rousette Genome Reveals Unexpected Features of Bat Antiviral Immunity.Cell. 2018 May 17;173(5):1098-1110.e18. doi: 10.1016/j.cell.2018.03.070. Epub 2018 Apr 26. Cell. 2018. PMID: 29706541 Free PMC article.
References
-
- Banerjee, A. K., S. A. Moyer, and D. P. Rhodes. 1974. Studies on the in vitro adenylation of RNA by vesicular stomatitis virus. Virology 61:547-558. - PubMed
-
- Barber, G. N. 2005. VSV-tumor selective replication and protein translation. Oncogene 24:7710-7719. - PubMed
-
- Both, G. W., Y. Furuichi, S. Muthukrishnan, and A. J. Shatkin. 1975. Ribosome binding to reovirus mRNA in protein synthesis requires 5′ terminal 7-methylguanosine. Cell 6:185-195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

