Preclinical model to test human papillomavirus virus (HPV) capsid vaccines in vivo using infectious HPV/cottontail rabbit papillomavirus chimeric papillomavirus particles
- PMID: 17005666
- PMCID: PMC1676303
- DOI: 10.1128/JVI.01583-06
Preclinical model to test human papillomavirus virus (HPV) capsid vaccines in vivo using infectious HPV/cottontail rabbit papillomavirus chimeric papillomavirus particles
Abstract
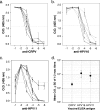
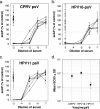
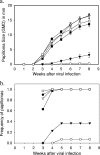
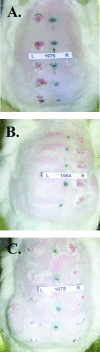
A human papillomavirus (HPV) vaccine consisting of virus-like particles (VLPs) was recently approved for human use. It is generally assumed that VLP vaccines protect by inducing type-specific neutralizing antibodies. Preclinical animal models cannot be used to test for protection against HPV infections due to species restriction. We developed a model using chimeric HPV capsid/cottontail rabbit papillomavirus (CRPV) genome particles to permit the direct testing of HPV VLP vaccines in rabbits. Animals vaccinated with CRPV, HPV type 16 (HPV-16), or HPV-11 VLPs were challenged with both homologous (CRPV capsid) and chimeric (HPV-16 capsid) particles. Strong type-specific protection was observed, demonstrating the potential application of this approach.
Figures
References
-
- Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. - PMC - PubMed
-
- Harper, D. M., E. L. Franco, C. Wheeler, D. G. Ferris, D. Jenkins, A. Schuind, T. Zahaf, B. Innis, P. Naud, N. S. De Carvalho, C. M. Roteli-Martins, J. Teixeira, M. M. Blatter, A. P. Korn, W. Quint, and G. Dubin. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757-1765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

