Evidence for CDK-dependent and CDK-independent functions of the murine gammaherpesvirus 68 v-cyclin
- PMID: 17005668
- PMCID: PMC1676255
- DOI: 10.1128/JVI.01722-06
Evidence for CDK-dependent and CDK-independent functions of the murine gammaherpesvirus 68 v-cyclin
Abstract
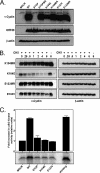
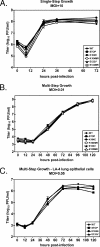
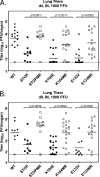
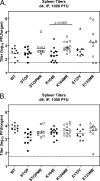
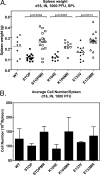
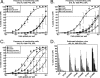
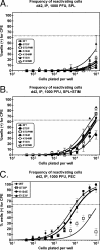
Gamma-2 herpesviruses encode homologues of mammalian D-type cyclins (v-cyclins), which likely function to manipulate the cell cycle, thereby providing a cellular environment conducive to virus replication and/or reactivation from latency. We have previously shown that the v-cyclin of murine gammaherpesvirus 68 is an oncogene that binds and activates cellular cyclin-dependent kinases (CDKs) and is required for efficient reactivation from latency. To determine the contribution of v-cyclin-mediated cell cycle regulation to the viral life cycle, recombinant viruses in which specific point mutations (E133V or K104E) were introduced into the v-cyclin open reading frame were generated, resulting in the disruption of CDK binding and activation. While in vitro growth of these mutant viruses was unaffected, lytic replication in the lungs following low-dose intranasal inoculation was attenuated for both mutants deficient in CDK binding as well as virus in which the entire v-cyclin open reading frame was disrupted by the insertion of a translation termination codon. This replication defect was not apparent in spleens of mice following intraperitoneal inoculation, suggesting a cell type- and/or route-specific dependence on v-cyclin-CDK interactions during the acute phase of virus infection. Notably, although a v-cyclin-null virus was highly attenuated for reactivation from latency, the E133V v-cyclin CDK-binding mutant exhibited only a modest defect in virus reactivation from splenocytes, and neither the E133V nor K104E v-cyclin mutants were compromised in reactivation from peritoneal exudate cells. Taken together, these data suggest that lytic replication and reactivation in vivo are differentially regulated by CDK-dependent and CDK-independent functions of v-cyclin, respectively.
Figures

References
-
- Adler, H., M. Messerle, and U. H. Koszinowski. 2003. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev. Med. Virol. 13:111-121. - PubMed
-
- Arellano, M., and S. Moreno. 1997. Regulation of CDK/cyclin complexes during the cell cycle. Int. J. Biochem. Cell Biol. 29:559-573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

