trans regulation of cap-independent translation by a viral subgenomic RNA
- PMID: 17005682
- PMCID: PMC1617300
- DOI: 10.1128/JVI.00991-06
trans regulation of cap-independent translation by a viral subgenomic RNA
Abstract
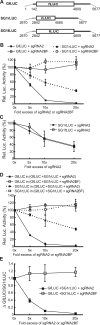
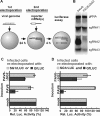
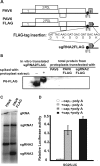
Many positive-strand RNA viruses generate 3'-coterminal subgenomic mRNAs to allow translation of 5'-distal open reading frames. It is unclear how viral genomic and subgenomic mRNAs compete with each other for the cellular translation machinery. Translation of the uncapped Barley yellow dwarf virus genomic RNA (gRNA) and subgenomic RNA1 (sgRNA1) is driven by the powerful cap-independent translation element (BTE) in their 3' untranslated regions (UTRs). The BTE forms a kissing stem-loop interaction with the 5' UTR to mediate translation initiation at the 5' end. Here, using reporter mRNAs that mimic gRNA and sgRNA1, we show that the abundant sgRNA2 inhibits translation of gRNA, but not sgRNA1, in vitro and in vivo. This trans inhibition requires the functional BTE in the 5' UTR of sgRNA2, but no translation of sgRNA2 itself is detectable. The efficiency of translation of the viral mRNAs in the presence of sgRNA2 is determined by proximity to the mRNA 5' end of the stem-loop that kisses the 3' BTE. Thus, the gRNA and sgRNA1 have "tuned" their expression efficiencies via the site in the 5' UTR to which the 3' BTE base pairs. We conclude that sgRNA2 is a riboregulator that switches off translation of replication genes from gRNA while permitting translation of structural genes from sgRNA1. These results reveal (i) a new level of control of subgenomic-RNA gene expression, (ii) a new role for a viral subgenomic RNA, and (iii) a new mechanism for RNA-mediated regulation of translation.
Figures



References
-
- Albarino, C. G., L. D. Eckerle, and L. A. Ball. 2003. The cis-acting replication signal at the 3′ end of Flock House virus RNA2 is RNA3-dependent. Virology 311:181-191. - PubMed
-
- Allen, E., S. Wang, and W. A. Miller. 1999. Barley yellow dwarf virus RNA requires a cap-independent translation sequence because it lacks a 5′ cap. Virology 253:139-144. - PubMed
-
- Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281-297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

