Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication
- PMID: 17005684
- PMCID: PMC1617282
- DOI: 10.1128/JVI.00678-06
Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication
Abstract
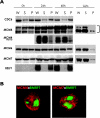
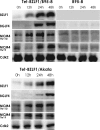
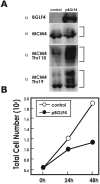
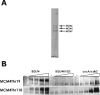
Induction of Epstein-Barr virus (EBV) lytic replication blocks chromosomal DNA replication notwithstanding an S-phase-like cellular environment with high cyclin-dependent kinase (CDK) activity. We report here that the phosphorylated form of MCM4, a subunit of the MCM complex essential for chromosomal DNA replication, increases with progression of lytic replication, Thr-19 and Thr-110 being CDK2/CDK1 targets whose phosphorylation inactivates MCM4-MCM6-MCM7 (MCM4-6-7) complex-associated DNA helicase. Expression of EBV-encoded protein kinase (EBV-PK) in HeLa cells caused phosphorylation of these sites on MCM4, leading to cell growth arrest. In vitro, the sites of MCM4 of the MCM4-6-7 hexamer were confirmed to be phosphorylated with EBV-PK, with the same loss of helicase activity as with CDK2/cyclin A. Introducing mutations in the N-terminal six Ser and Thr residues of MCM4 reduced the inhibition by CDK2/cyclin A, while EBV-PK inhibited the helicase activities of both wild-type and mutant MCM4-6-7 hexamers, probably since EBV-PK can phosphorylate MCM6 and another site(s) of MCM4 in addition to the N-terminal residues. Therefore, phosphorylation of the MCM complex by redundant actions of CDK and EBV-PK during lytic replication might provide one mechanism to block chromosomal DNA replication in the infected cells through inactivation of DNA unwinding by the MCM4-6-7 complex.
Figures

References
-
- Aparicio, O. M., D. M. Weinstein, and S. P. Bell. 1997. Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91:59-69. - PubMed
-
- Bailis, J. M., and S. L. Forsburg. 2004. MCM proteins: DNA damage, mutagenesis and repair. Curr. Opin. Genet. Dev. 14:17-21. - PubMed
-
- Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

