The amino terminus of the herpes simplex virus 1 protein Vhs mediates membrane association and tegument incorporation
- PMID: 17005689
- PMCID: PMC1617289
- DOI: 10.1128/JVI.00744-06
The amino terminus of the herpes simplex virus 1 protein Vhs mediates membrane association and tegument incorporation
Abstract
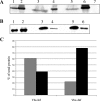
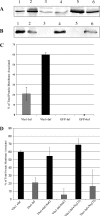
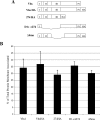
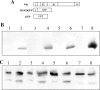
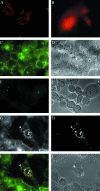
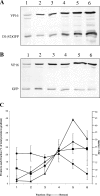
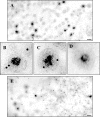
Assembly of herpes simplex viruses (HSV) is a poorly understood process involving multiple redundant interactions between large number of tegument and envelope proteins. We have previously shown (G. E. Lee, G. A. Church, and D. W. Wilson, J. Virol. 77:2038-2045, 2003) that the virion host shutoff (Vhs) tegument protein is largely insoluble in HSV-infected cells and is also stably associated with membranes. Here we demonstrate that both insolubility and stable membrane binding are stimulated during the course of an HSV infection. Furthermore, we have found that the amino-terminal 42 residues of Vhs are sufficient to mediate membrane association and tegument incorporation when fused to a green fluorescent protein (GFP) reporter. Particle incorporation correlates with sorting to cytoplasmic punctate structures that may correspond to sites of HSV assembly. We conclude that the amino terminus of Vhs mediates targeting to sites of HSV assembly and to the viral tegument.
Figures



Similar articles
-
A subpopulation of tegument protein vhs localizes to detergent-insoluble lipid rafts in herpes simplex virus-infected cells.J Virol. 2003 Feb;77(3):2038-45. doi: 10.1128/jvi.77.3.2038-2045.2003. J Virol. 2003. PMID: 12525638 Free PMC article.
-
Assembly of infectious Herpes simplex virus type 1 virions in the absence of full-length VP22.J Virol. 2000 Nov;74(21):10041-54. doi: 10.1128/jvi.74.21.10041-10054.2000. J Virol. 2000. PMID: 11024133 Free PMC article.
-
Incorporation of the herpes simplex virus type 1 tegument protein VP22 into the virus particle is independent of interaction with VP16.Virology. 2007 Dec 20;369(2):263-80. doi: 10.1016/j.virol.2007.07.020. Epub 2007 Sep 20. Virology. 2007. PMID: 17888478
-
Early shutoff of host protein synthesis in cells infected with herpes simplex viruses.Acta Virol. 2001;45(5-6):269-77. Acta Virol. 2001. PMID: 12083325 Review.
-
[Visualization of viruses in living cells].Uirusu. 2008 Dec;58(2):117-24. doi: 10.2222/jsv.58.117. Uirusu. 2008. PMID: 19374190 Review. Japanese.
Cited by
-
UL36p is required for efficient transport of membrane-associated herpes simplex virus type 1 along microtubules.J Virol. 2008 Aug;82(15):7388-94. doi: 10.1128/JVI.00225-08. Epub 2008 May 21. J Virol. 2008. PMID: 18495763 Free PMC article.
-
Virion incorporation of the herpes simplex virus type 1 tegument protein VP22 is facilitated by trans-Golgi network localization and is independent of interaction with glycoprotein E.Virology. 2010 Sep 15;405(1):176-92. doi: 10.1016/j.virol.2010.06.007. Epub 2010 Jun 26. Virology. 2010. PMID: 20580397 Free PMC article.
-
Time-dependent transformation of the herpesvirus tegument.J Virol. 2009 Aug;83(16):8082-9. doi: 10.1128/JVI.00777-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19494000 Free PMC article.
-
Characterization of subcellular localization of duck enteritis virus UL51 protein.Virol J. 2009 Jul 3;6:92. doi: 10.1186/1743-422X-6-92. Virol J. 2009. PMID: 19575796 Free PMC article.
-
Herpes Simplex Virus Capsid Localization to ESCRT-VPS4 Complexes in the Presence and Absence of the Large Tegument Protein UL36p.J Virol. 2016 Jul 27;90(16):7257-7267. doi: 10.1128/JVI.00857-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252536 Free PMC article.
References
-
- Berthomme, H., B. Jacquemont, and A. Epstein. 1993. The pseudorabies virus host-shutoff homolog gene: nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology 193:1028-1032. - PubMed
-
- Chi, J. H., C. A. Harley, A. Mukhopadhyay, and D. W. Wilson. 2005. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids. J. Gen. Virol. 86:253-261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

