The amino terminus of the herpes simplex virus 1 protein Vhs mediates membrane association and tegument incorporation
- PMID: 17005689
- PMCID: PMC1617289
- DOI: 10.1128/JVI.00744-06
The amino terminus of the herpes simplex virus 1 protein Vhs mediates membrane association and tegument incorporation
Abstract
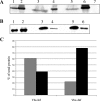
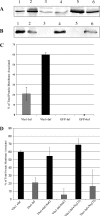
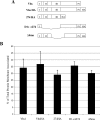
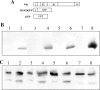
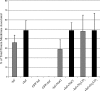
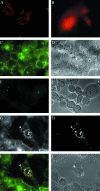
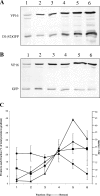
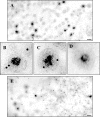
Assembly of herpes simplex viruses (HSV) is a poorly understood process involving multiple redundant interactions between large number of tegument and envelope proteins. We have previously shown (G. E. Lee, G. A. Church, and D. W. Wilson, J. Virol. 77:2038-2045, 2003) that the virion host shutoff (Vhs) tegument protein is largely insoluble in HSV-infected cells and is also stably associated with membranes. Here we demonstrate that both insolubility and stable membrane binding are stimulated during the course of an HSV infection. Furthermore, we have found that the amino-terminal 42 residues of Vhs are sufficient to mediate membrane association and tegument incorporation when fused to a green fluorescent protein (GFP) reporter. Particle incorporation correlates with sorting to cytoplasmic punctate structures that may correspond to sites of HSV assembly. We conclude that the amino terminus of Vhs mediates targeting to sites of HSV assembly and to the viral tegument.
Figures



References
-
- Berthomme, H., B. Jacquemont, and A. Epstein. 1993. The pseudorabies virus host-shutoff homolog gene: nucleotide sequence and comparison with alphaherpesvirus protein counterparts. Virology 193:1028-1032. - PubMed
-
- Chi, J. H., C. A. Harley, A. Mukhopadhyay, and D. W. Wilson. 2005. The cytoplasmic tail of herpes simplex virus envelope glycoprotein D binds to the tegument protein VP22 and to capsids. J. Gen. Virol. 86:253-261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

