Production of infectious hepatitis C virus by well-differentiated, growth-arrested human hepatoma-derived cells
- PMID: 17005703
- PMCID: PMC1617281
- DOI: 10.1128/JVI.01059-06
Production of infectious hepatitis C virus by well-differentiated, growth-arrested human hepatoma-derived cells
Abstract
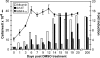
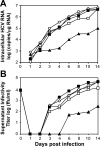
Dimethyl sulfoxide (DMSO) has been shown to induce the differentiation of primary hepatocytes in vitro. When actively dividing poorly differentiated human hepatoma-derived (Huh7) cells were cultured in the presence of 1% DMSO, cells became cytologically differentiated and transitioned into a nondividing state, characterized by the induction of hepatocyte-specific genes. Moreover, these cells were highly permissive for acute hepatitis C virus (HCV) infection, and persistent long term infection of these cultures could also be achieved. As HCV naturally replicates in highly differentiated nondividing human hepatocytes, this system may more accurately mimic the conditions under which HCV replicates in vivo than previous models using poorly differentiated rapidly dividing hepatoma cells.
Figures


Similar articles
-
Human serum leads to differentiation of human hepatoma cells, restoration of very-low-density lipoprotein secretion, and a 1000-fold increase in HCV Japanese fulminant hepatitis type 1 titers.Hepatology. 2013 Dec;58(6):1907-17. doi: 10.1002/hep.26566. Epub 2013 Oct 17. Hepatology. 2013. PMID: 23775894
-
Low perforin expression of early differentiated HCV-specific CD8+ T cells limits their hepatotoxic potential.J Hepatol. 2012 Jul;57(1):9-16. doi: 10.1016/j.jhep.2012.02.030. Epub 2012 Mar 14. J Hepatol. 2012. PMID: 22425625
-
Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes.Gastroenterology. 2010 Oct;139(4):1355-64. doi: 10.1053/j.gastro.2010.06.058. Epub 2010 Jul 1. Gastroenterology. 2010. PMID: 20600021
-
Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system.Gastroenterology. 2007 Nov;133(5):1649-59. doi: 10.1053/j.gastro.2007.09.017. Epub 2007 Sep 16. Gastroenterology. 2007. PMID: 17983809
-
Primary hepatocyte culture supports hepatitis C virus replication: a model for infection-associated hepatocarcinogenesis.Hepatology. 2010 Jun;51(6):1922-32. doi: 10.1002/hep.23616. Hepatology. 2010. PMID: 20512986
Cited by
-
In vitro systems for the study of hepatitis C virus infection.Int J Hepatol. 2012;2012:292591. doi: 10.1155/2012/292591. Epub 2012 Sep 27. Int J Hepatol. 2012. PMID: 23056952 Free PMC article.
-
A small-molecule inhibitor of hepatitis C virus infectivity.Antimicrob Agents Chemother. 2014;58(1):386-96. doi: 10.1128/AAC.02083-13. Epub 2013 Oct 28. Antimicrob Agents Chemother. 2014. PMID: 24165192 Free PMC article.
-
Claudin-6 and Occludin Natural Variants Found in a Patient Highly Exposed but Not Infected with Hepatitis C Virus (HCV) Do Not Confer HCV Resistance In Vitro.PLoS One. 2015 Nov 12;10(11):e0142539. doi: 10.1371/journal.pone.0142539. eCollection 2015. PLoS One. 2015. PMID: 26561856 Free PMC article.
-
Rapid Rescue of Goose Astrovirus Genome via Red/ET Assembly.Food Environ Virol. 2024 Sep;16(3):297-306. doi: 10.1007/s12560-024-09593-4. Epub 2024 Apr 6. Food Environ Virol. 2024. PMID: 38582780
-
Potential treatment options and future research to increase hepatitis C virus treatment response rate.Hepat Med. 2010 Oct;2010(2):125-145. doi: 10.2147/HMER.S7193. Hepat Med. 2010. PMID: 21331152 Free PMC article.
References
-
- Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. - PubMed
-
- Alter, M. J. 1999. Hepatitis C virus infection in the United States. J. Hepatol. 31(Suppl. 1):88-91. - PubMed
-
- Alter, M. J. 2002. Prevention of spread of hepatitis C. Hepatology 36:S93-S98. - PubMed
-
- Aninat, C., A. Piton, D. Glaise, T. Le Charpentier, S. Langouet, F. Morel, C. Guguen-Guillouzo, and A. Guillouzo. 2006. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab. Dispos. 34:75-83. - PubMed
-
- Arterburn, L. M., J. Zurlo, J. D. Yager, R. M. Overton, and A. H. Heifetz. 1995. A morphological study of differentiated hepatocytes in vitro. Hepatology 22:175-187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

