Nuclear import defect of human immunodeficiency virus type 1 DNA flap mutants is not dependent on the viral strain or target cell type
- PMID: 17005705
- PMCID: PMC1617309
- DOI: 10.1128/JVI.00974-06
Nuclear import defect of human immunodeficiency virus type 1 DNA flap mutants is not dependent on the viral strain or target cell type
Abstract
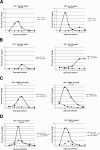
We have previously established, using human immunodeficiency virus type 1 (HIV-1) strain LAI, that the HIV-1 central DNA Flap acts as a cis determinant of viral genome nuclear import. Although the impact of the DNA Flap on nuclear import has already found numerous independent confirmations in the context of lentivirus vectors, it has been claimed that it may be nonessential for infectious virus strains LAI, YU-2 (J. D. Dvorin et al., J. Virol. 76:12087-12096, 2002), HXB2, and NL4-3 (A. Limon et al., J. Virol. 76:12078-12086, 2002). We conducted a detailed analysis of virus infectivity using the provirus clones provided by the authors and analogous target cells. In contrast to published data, our results show that all cPPT mutant viruses exhibit reduced infectivity corresponding to a nuclear import defect irrespective of the viral genetic background or target cell.
Figures



References
-
- Baekelandt, V., A. Claeys, K. Eggermont, E. Lauwers, B. De Strooper, B. Nuttin, and Z. Debyser. 2002. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum. Gene Ther. 13:841-853. - PubMed
-
- Barry, S. C., B. Harder, M. Brzezinski, L. Y. Flint, J. Seppen, and W. R. Osborne. 2001. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum. Gene Ther. 12:1103-1108. - PubMed
-
- Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

