Nucleolar trafficking is essential for nuclear export of intronless herpesvirus mRNA
- PMID: 17005724
- PMCID: PMC1622798
- DOI: 10.1073/pnas.0604890103
Nucleolar trafficking is essential for nuclear export of intronless herpesvirus mRNA
Abstract
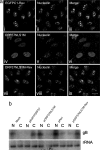
The nucleolus is the largest subnuclear structure and is plurifunctional in nature. Here, we demonstrate that nucleolar localization of a key herpesvirus regulatory protein is essential for its role in virus mRNA nuclear export. The herpesvirus saimiri ORF57 protein is a nucleocytoplasmic shuttle protein that is conserved in all herpesviruses and orchestrates the nuclear export of viral intronless mRNAs. We demonstrate that expression of the ORF57 protein induces nucleolar redistribution of human TREX (transcription/export) proteins that are involved in mRNA nuclear export. Moreover, we describe a previously unidentified nucleolar localization signal within ORF57 that is composed of two distinct nuclear localization signals. Intriguingly, point mutations that ablate ORF57 nucleolar localization lead to a failure of ORF57-mediated viral mRNA nuclear export. Furthermore, nucleolar retargeting of the ORF57 mutant was achieved by the incorporation of the HIV-1 Rev nucleolar localization signal, and analysis demonstrated that this modification was sufficient to restore viral mRNA nuclear export. This finding represents a unique and fundamental role for the nucleolus in nuclear export of viral mRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

