Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers
- PMID: 17005801
- PMCID: PMC1610083
- DOI: 10.1128/AAC.00247-06
Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers
Abstract
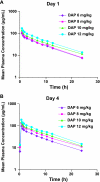
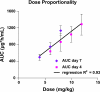
Daptomycin, a novel lipopeptide, is bactericidal against a broad range of gram-positive strains, including methicillin- (MRSA) and vancomycin-resistant Staphylococcus aureus. Daptomycin is approved at 4 mg/kg of body weight given intravenously once daily for the treatment of complicated skin and skin structure infections and at 6 mg/kg for the treatment of S. aureus bloodstream infections (bacteremia), including right-sided endocarditis caused by methicillin-susceptible S. aureus and MRSA. The present study was designed to evaluate the multiple-dose pharmacokinetics and safety of daptomycin at doses of 6 to 12 mg/kg in healthy volunteers. Three cohorts of 12 subjects each were given daptomycin (10 mg/kg) or placebo once daily for 14 days, daptomycin (12 mg/kg) or placebo once daily for 14 days, or daptomycin (6 or 8 mg/kg) once daily for 4 days. Daptomycin produced dose-proportional increases in the area under the plasma concentration-time curve and in trough daptomycin levels and nearly dose-proportional increases in peak daptomycin concentrations. Other pharmacokinetic parameters measured on day 1 and at steady state were independent of the dose, including the half-life (approximately 8 h), weight-normalized plasma clearance (9 to 10 ml/h/kg), and volume of distribution (approximately 100 ml/kg). Plasma protein binding was 90% to 93% and was independent of the daptomycin concentration. Daptomycin did not produce electrocardiographic abnormalities or electrophysiological evidence of muscle or nerve toxicity. Daptomycin was well tolerated in subjects dosed with up to 12 mg/kg intravenously for 14 days. Doses of daptomycin higher than 6 mg/kg once daily may be considered in further studies to evaluate the safety and efficacy of daptomycin in difficult-to-treat infections.
Figures
References
-
- Arbeit, R. D., D. Maki, F. P. Tally, E. Campanaro, and B. I. Eisenstein. 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673-1681. - PubMed
-
- Carpenter, C. F., and H. F. Chambers. 2004. Daptomycin: another novel agent for treating infections due to drug-resistant Gram-positive pathogens. Clin. Infect. Dis. 38:994-1000. - PubMed
-
- Cubist Pharmaceuticals. 2003. Cubicin (daptomycin for injection) prescribing information. Cubist Pharmaceuticals, Lexington, Mass.
-
- Cubist Pharmaceuticals. 2006. Cubicin (daptomycin for injection) prescribing information. Cubist Pharmaceuticals, Lexington, Mass.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

