Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance
- PMID: 17005804
- PMCID: PMC1610057
- DOI: 10.1128/AAC.00556-06
Biofilm formation by the emerging fungal pathogen Trichosporon asahii: development, architecture, and antifungal resistance
Abstract
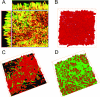
Trichosporon asahii is the most common cause of fatal disseminated trichosporonosis, frequently associated with indwelling medical devices. Despite the use of antifungal drugs to treat trichosporonosis, infection is often persistent and is associated with high mortality. This drove our interest in evaluating the capability of T. asahii to form a biofilm on biomaterial-representative polystyrene surfaces through the development and optimization of a reproducible T. asahii-associated biofilm model. Time course analyses of viable counts and a formazan salt reduction assay, as well as microscopy studies, revealed that biofilm formation by T. asahii occurred in an organized fashion through four distinct developmental phases: initial adherence of yeast cells (0 to 2 h), germination and microcolony formation (2 to 4 h), filamentation (4 to 6 h), and proliferation and maturation (24 to 72 h). Scanning electron microscopy and confocal scanning laser microscopy revealed that mature T. asahii biofilms (72-h) displayed a complex, heterogeneous three-dimensional structure, consisting of a dense network of metabolically active yeast cells and hyphal elements completely embedded within exopolymeric material. Antifungal susceptibility testing demonstrated a remarkable rise in the MICs of sessile T. asahii cells against clinically used amphotericin B, caspofungin, voriconazole, and fluconazole compared to their planktonic counterparts. In particular, T. asahii biofilms were up to 16,000 times more resistant to voriconazole, the most active agent against planktonic cells (MIC, 0.06 microg/ml). Our results suggest that the ability of T. asahii to form a biofilm may be a major factor in determining persistence of the infection in spite of in vitro susceptibility of clinical isolates.
Figures


References
-
- Abdala, E., R. I. Lopes, C. N. Chaves, E. M. Heins-Vaccari, and M. A. Shikanai-Yasuda. 2005. Trichosporon asahii fatal infection in a non-neutropenic patient after orthotopic liver transplantation. Transpl. Infect. Dis. 7:162-165. - PubMed
-
- Armitage, G. C. 2004. Basic features of biofilms—why are they difficult therapeutic targets? Ann. R. Australas. Coll. Dent. Surg. 17:30-34. - PubMed
-
- Chandra, J., G. Zhou, and M. A. Ghannoum. 2005. Fungal biofilms and antimycotics. Curr. Drug Targets 6:887-894. - PubMed
-
- Chowdhary, A., S. Ahmad, Z. U. Khan, D. C. Doval, and H. S. Randhawa. 2004. Trichosporon asahii as an emerging etiologic agent of disseminated trichosporonosis: a case report and an update. Ind. J. Med. Microbiol. 22:16-22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

