Effect of PEX, a noncatalytic metalloproteinase fragment with integrin-binding activity, on experimental Chlamydophila pneumoniae infection
- PMID: 17005805
- PMCID: PMC1610071
- DOI: 10.1128/AAC.00108-06
Effect of PEX, a noncatalytic metalloproteinase fragment with integrin-binding activity, on experimental Chlamydophila pneumoniae infection
Abstract
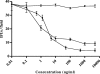
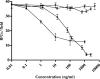
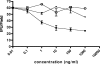
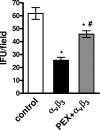
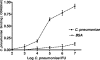
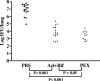
Chlamydophila pneumoniae is a pathogen that is involved in acute and chronic respiratory infections and that is associated with asthma and coronary artery diseases. In this study, we evaluated the effects of PEX, a noncatalytic metalloproteinase fragment with integrin-binding activity, against experimental infections caused by C. pneumoniae. Moreover, we investigated the relationships between C. pneumoniae and alpha(v)beta(3) integrin functions in order to explain the possible mechanism of action of PEX both in vitro and in vivo. For the in vitro experiments, HeLa cells were infected with C. pneumoniae and treated with either PEX or azithromycin. The results obtained with PEX were not significantly different (P > 0.05) from those achieved with azithromycin. Similar results were also obtained in a lung infection model. Male C57BL/J6 mice inoculated intranasally with 10(6) inclusion-forming units of C. pneumoniae were treated with either PEX or azithromycin plus rifampin. Infected mice treated with PEX showed a marked decrease in C. pneumoniae counts versus those for the controls; this finding did not differ significantly (P > 0.05) from the results observed for the antibiotic-treated group. Integrin alpha(v)beta(3) plays an important role in C. pneumoniae infection. Blockage of integrin activation led to a significant inhibition of C. pneumoniae infection in HeLa cells. Moreover, CHO(DHFR) alpha(v)beta(3)-expressing cells were significantly (P < 0.001) more susceptible to C. pneumoniae infection than CHO(DHFR) cells. These results offer new perspectives on the treatment of C. pneumoniae infection and indicate that alpha(v)beta(3) could be a promising target for new agents developed for activity against this pathogen.
Figures

Similar articles
-
Effect of azithromycin on murine arteriosclerosis exacerbated by Chlamydia pneumoniae.J Infect Dis. 2001 Jan 15;183(2):232-238. doi: 10.1086/317941. Epub 2000 Dec 13. J Infect Dis. 2001. PMID: 11120929
-
Glucocorticoids increase in vitro and in vivo activities of antibiotics against Chlamydophila pneumoniae.Antimicrob Agents Chemother. 2004 Dec;48(12):4878-81. doi: 10.1128/AAC.48.12.4878-4881.2004. Antimicrob Agents Chemother. 2004. PMID: 15561871 Free PMC article.
-
Toll-like receptors 2 and 4 do not contribute to clearance of Chlamydophila pneumoniae in mice, but are necessary for the release of monokines.Immunobiology. 2004;209(8):599-608. doi: 10.1016/j.imbio.2004.08.003. Immunobiology. 2004. PMID: 15638128
-
[Choice of antimicrobial drug for infections caused by Chlamydia trachomatis and Chlamydophila pneumoniae].Acta Med Croatica. 2004;58(4):329-33. Acta Med Croatica. 2004. PMID: 15700690 Review. Croatian.
-
Chlamydia pneumoniae infections in mouse models: relevance for atherosclerosis research.Cardiovasc Res. 2005 Feb 1;65(2):317-27. doi: 10.1016/j.cardiores.2004.09.031. Cardiovasc Res. 2005. PMID: 15639470 Review.
References
-
- Al-Fakhri, N., J. Wilhelm, M. Hahn, M. Heidt, F. W. Hehrlein, A. M. Endisch, T. Hupp, S. M. Cherian, Y. V. Bobryshev, R. S. Lord, and N. Katz. 2003. Increased expression of disintegrin-metalloproteinases ADAM-15 and ADAM-9 following upregulation of integrins alpha5beta1 and alphavbeta3 in atherosclerosis. J. Cell. Biochem. 89:808-823. - PubMed
-
- Bello, L., G. Carrabba, C. Giussani, V. Lucini, F. Cerutti, F. Scaglione, J. Landré, G. Tomei, R. Villani, R. S. Carroll, P. M. Black, and A. Bikfalvi. 2001. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in-vivo. Cancer Res. 61:7501-7506. - PubMed
-
- Brooks, P. C., S. Silletti, T. L. von Schalscha, M. Friedlander, and D. A. Cheresh. 1998. Disruption of angiogenesis by PEX a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 92:391-400. - PubMed
-
- Coombes, B. K., and J. B. Mahony. 2002. Identification of MEK- and phosphoinositide 3-kinase-dependent signalling as essential events during Chlamydia pneumoniae invasion of Hep2 cells. Cell. Microbiol. 4:447-460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

