Mechanism of active renal tubular efflux of tenofovir
- PMID: 17005808
- PMCID: PMC1610069
- DOI: 10.1128/AAC.00251-06
Mechanism of active renal tubular efflux of tenofovir
Abstract
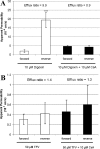
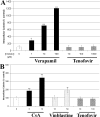
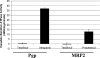
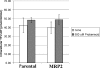
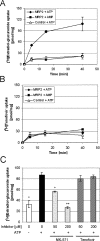
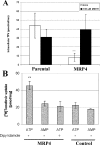
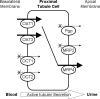
Tenofovir (TFV) undergoes renal elimination by a combination of glomerular filtration and active tubular secretion. While transporter-mediated uptake of TFV from the blood into proximal-tubule cells has been well characterized, comparatively little is known about the efflux system responsible for transporting TFV into the lumen during active tubular secretion. Therefore, members of the ATP-binding cassette family of efflux pumps expressed at the apical side of proximal-tubule cells were studied for the ability to transport TFV. Studies in multiple independent in vitro systems show TFV not to be a substrate for P glycoprotein (Pgp) or multidrug resistance protein type 2 (MRP2). In contrast to Pgp and MRP2, TFV was observed to be a substrate for MRP4. TFV accumulated to fivefold lower levels in MRP4-overexpressing cells, and its accumulation could be increased by an MRP inhibitor. Furthermore, MRP4-overexpressing cells were found to be 2.0- to 2.5-fold less susceptible to cytotoxicity caused by TFV. ATP-dependent uptake of TFV was observed in membrane vesicles containing MRP4 but not in vesicles lacking the transporter. On the basis of these and previous results, the molecular transport pathway for the active tubular secretion of TFV through renal proximal-tubule cells involves uptake from the blood mediated by human organic anion transporters 1 and 3 and efflux into urine by MRP4. A detailed understanding of the molecular mechanism of TFV active tubular secretion will facilitate the assessment of potential renal drug-drug interactions with coadministered agents.
Figures
References
-
- Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. - PMC - PubMed
-
- Bonate, P. L., K. Reith, and S. Weir. 1998. Drug interactions at the renal level. Implications for drug development. Clin. Pharmacokinet. 34:375-404. - PubMed
-
- Chen, Z. S., K. Lee, S. Walther, R. B. Raftogianis, M. Kuwano, H. Zeng, and G. D. Kruh. 2002. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 62:3144-3150. - PubMed
-
- Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. M. Hitchcock. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 54:37-45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

