Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells
- PMID: 17005819
- PMCID: PMC1610064
- DOI: 10.1128/AAC.00593-06
Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells
Abstract
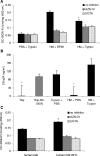
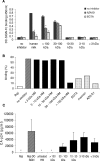
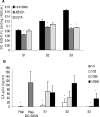
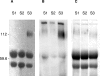
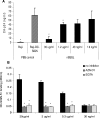
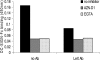
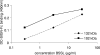
A wide range of pathogens, including human immunodeficiency virus type 1 (HIV-1), hepatitis C virus, Ebola virus, cytomegalovirus, dengue virus, Mycobacterium, Leishmania, and Helicobacter pylori, can interact with dendritic cell (DC)-specific ICAM3-grabbing nonintegrin (DC-SIGN), expressed on DCs and a subset of B cells. More specifically, the interaction of the gp120 envelope protein of HIV-1 with DC-SIGN can facilitate the transfer of virus to CD4+ T lymphocytes in trans and enhance infection. We have previously demonstrated that a multimeric LeX component in human milk binds to DC-SIGN, preventing HIV-1 from interacting with this receptor. Biochemical analysis reveals that the compound is heat resistant, trypsin sensitive, and larger than 100 kDa, indicating a specific glycoprotein as the inhibitory compound. By testing human milk from three different mothers, we found the levels of DC-SIGN binding and viral inhibition to vary between samples. Using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and matrix-assisted laser desorption ionization analysis, we identified bile salt-stimulated lipase (BSSL), a Lewis X (LeX)-containing glycoprotein found in human milk, to be the major variant protein between the samples. BSSL isolated from human milk bound to DC-SIGN and inhibited the transfer of HIV-1 to CD4+ T lymphocytes. Two BSSL isoforms isolated from the same human milk sample showed differences in DC-SIGN binding, illustrating that alterations in the BSSL forms explain the differences observed. These results indicate that variations in BSSL lead to alterations in LeX expression by the protein, which subsequently alters the DC-SIGN binding capacity and the inhibitory effect on HIV-1 transfer. Identifying the specific molecular interaction between the different forms may aid in the future design of antimicrobial agents.
Figures
Similar articles
-
Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes.J Clin Invest. 2005 Nov;115(11):3256-64. doi: 10.1172/JCI25105. Epub 2005 Oct 20. J Clin Invest. 2005. PMID: 16239964 Free PMC article.
-
Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes.Virology. 2009 Sep 1;391(2):203-11. doi: 10.1016/j.virol.2009.06.011. Virology. 2009. PMID: 19682628
-
Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells.J Virol. 2006 Mar;80(6):2949-57. doi: 10.1128/JVI.80.6.2949-2957.2006. J Virol. 2006. PMID: 16501104 Free PMC article.
-
DC-SIGN points the way to a novel mechanism for HIV-1 transmission.MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review.
-
DC-SIGN: binding receptors for hepatitis C virus.Chin Med J (Engl). 2004 Sep;117(9):1395-400. Chin Med J (Engl). 2004. PMID: 15377434 Review.
Cited by
-
Binding of human milk to pathogen receptor DC-SIGN varies with bile salt-stimulated lipase (BSSL) gene polymorphism.PLoS One. 2011 Feb 28;6(2):e17316. doi: 10.1371/journal.pone.0017316. PLoS One. 2011. PMID: 21386960 Free PMC article.
-
HIV-1 autologous antibody neutralization associates with mother to child transmission.PLoS One. 2013 Jul 17;8(7):e69274. doi: 10.1371/journal.pone.0069274. Print 2013. PLoS One. 2013. PMID: 23874931 Free PMC article.
-
Immunology of pediatric HIV infection.Immunol Rev. 2013 Jul;254(1):143-69. doi: 10.1111/imr.12074. Immunol Rev. 2013. PMID: 23772619 Free PMC article. Review.
-
Impaired dendritic cell maturation and IL-10 production following H. pylori stimulation in gastric cancer patients.Appl Microbiol Biotechnol. 2012 Oct;96(1):211-20. doi: 10.1007/s00253-012-4034-z. Epub 2012 Apr 13. Appl Microbiol Biotechnol. 2012. PMID: 22526791 Free PMC article.
-
Human DC-SIGN binds specific human milk glycans.Biochem J. 2016 May 15;473(10):1343-53. doi: 10.1042/BCJ20160046. Epub 2016 Mar 14. Biochem J. 2016. PMID: 26976925 Free PMC article.
References
-
- Baba, T., D. Downs, K. W. Jackson, J. Tang, and C. S. Wang. 1991. Structure of human milk bile salt activated lipase. Biochemistry 30:500-510. - PubMed
-
- Bergman, M. P., A. Engering, H. H. Smits, S. J. van Vliet, A. A. van Bodegraven, H. P. Wirth, M. L. Kapsenberg, C. M. Vandenbroucke-Grauls, Y. van Kooyk, and B. J. Appelmelk. 2004. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200:979-990. - PMC - PubMed
-
- Blackberg, L., K. A. Angquist, and O. Hernell. 1987. Bile-salt-stimulated lipase in human milk: evidence for its synthesis in the lactating mammary gland. FEBS Lett. 217:37-41. - PubMed
-
- Blackberg, L., and O. Hernell. 1981. The bile-salt-stimulated lipase in human milk. Purification and characterization. Eur. J. Biochem. 116:221-225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

